Arch Iran Med. 28(2):112-123.
doi: 10.34172/aim.33505
Systematic Review
From Conventional Detection to Point-of-care Tests (POCT) Method for Pediatric Respiratory Infections Diagnosis: A Systematic Review
Reza Azizian Conceptualization, Formal analysis, Resources, Writing – original draft, 1, 2, * 
Setareh Mamishi Project administration, 1
Erfaneh Jafari Resources, Visualization, Writing – review & editing, 1, 2 
Mohammad Reza Mohammadi Methodology, 3
Fereshteh Heidari Tajabadi Methodology, 4
Babak Pourakbari Conceptualization, Project administration, Supervision, Validation, Writing – review & editing, 1, * 
Author information:
1Pediatric Infectious Diseases Research Center (PIDRC), Tehran University of Medical Sciences, Tehran, Iran
2Biomedical Innovation and Start-up Student Association (Biomino), Tehran University of Medical Sciences, Tehran, Iran
3Department of Bacteriology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
4Department of Microbiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Bacterial respiratory infections pose significant health risks to children, particularly infants susceptible to upper respiratory tract infections (URTIs). The COVID-19 pandemic has further exacerbated the prevalence of these infections, with pathogens such as Mycoplasma pneumoniae, Streptococcus pneumoniae, Legionella pneumophila, Staphylococcus aureus, Haemophilus influenzae, and Klebsiella species commonly implicated in pediatric cases. The critical need for accurate and timely detection of these bacterial agents has highlighted the importance of advanced diagnostic techniques, including multiplex real-time PCR, in clinical practice. Multiplex real-time polymerase chain reaction (PCR) offers several advantages, including rapid results, high sensitivity, and specificity. By accelerating the diagnostic process, this approach enables early intervention and targeted treatment, ultimately improving patient outcomes. In addition to PCR technologies, rapid and point-of-care testing (POCT) play a crucial role in the prompt diagnosis of bacterial respiratory infections. These tests are designed to be user-friendly, sensitive, and deliver quick results, making them particularly valuable in urgent clinical settings. POCT tests are often categorized into two main groups: those aimed at determining the cause of infection and those focused on confirming the presence of specific pathogens. By utilizing POCT, healthcare providers can make rapid and informed treatment decisions, leading to more effective management of bacterial respiratory infections in children. As the medical community continues to explore innovative diagnostic approaches, the integration of molecular and rapid testing methods offers significant promise in the realm of bacterial respiratory infections. By adopting these cutting-edge technologies, healthcare professionals can enhance their ability to accurately diagnose these infections, tailor treatment strategies, and ultimately improve patient care.
Keywords: Molecular diagnosis, Pediatric infections, POCT, Point-of-care testing, Respiratory infections
Copyright and License Information
© 2025 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Azizian R, Mamishi S, Jafari E, Mohammadi MR, Heidari Tajabadi F, Pourakbari B. From conventional detection to point-of-care tests (POCT) method for pediatric respiratory infections diagnosis: a systematic review. Arch Iran Med. 2025;28(2):112-123. doi: 10.34172/aim.33505
Introduction
Molecular and conventional detection methods play a crucial role in the diagnosis and management of pediatric respiratory infections. Conventional methods, such as culture-based techniques, have long been the standard approach for identifying pathogens causing respiratory infections in children. However, these methods are time-consuming and may lack sensitivity, particularly for fastidious organisms.1 In contrast, molecular methods, including polymerase chain reaction (PCR) and nucleic acid amplification tests, offer rapid and accurate detection of a wide range of respiratory pathogens in pediatric patients.2
These molecular techniques have revolutionized the field of diagnostic microbiology by providing sensitive and specific identification of viruses, bacteria, and fungi causing respiratory infections. The rapid and accurate diagnosis facilitated by molecular methods allows for appropriate and timely treatment, reduces unnecessary antibiotic use, and aids in infection control measures to prevent the spread of pathogens within healthcare settings.3
The increasing prevalence of antibiotic-resistant bacteria has further underscored the importance of accurate and rapid diagnostic methods. More than 70% of uncertain diagnoses and prescriptions of antibiotics contribute to the rising antibacterial resistance in children.4 While clinical examination may suffice for diagnosing upper respiratory tract infections (URTIs), lower respiratory tract infections often present diagnostic challenges, necessitating more advanced techniques.5
The effectiveness of microbiological tests is influenced by various factors, including the origin of the sample, the concentrations and pathogenic potential of different organisms, and the impact of previous antibiotic therapy.6,7 Common identification methods include X-ray imaging, culturing, gram staining, and biochemical and molecular assays.5 For comprehensive diagnosis of respiratory infections, a combination of tests is often necessary, including blood culture, sputum microscopy and culture, urinary antigen tests for specific pathogens, and serology.8
As we delve deeper into the various diagnostic approaches for pediatric respiratory infections, it becomes clear that a multi-faceted strategy, incorporating both conventional and molecular methods, is essential for accurate diagnosis and effective treatment. The following sections will explore in detail the specific bacterial pathogens involved in pediatric respiratory infections, the advantages and limitations of different diagnostic techniques, and the promising role of point-of-care testing (POCT) in improving patient outcomes.
Bacterial Respiratory Infections in Children
Bacterial respiratory infections are a significant health concern for children, potentially leading to severe complications if left untreated. These infections primarily affect the upper or lower respiratory tract, causing symptoms such as coughing, fever, and difficulty breathing. The most common bacterial pathogens implicated in pediatric respiratory infections include Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus.9-11
Transmission and Risk Factors
These bacteria are typically transmitted through respiratory droplets expelled during coughing, sneezing, or close contact with infected individuals. Several factors increase the risk of contracting these infections, including: poor hygiene practices, crowded living conditions, weakened immune systems, exposure to environmental pollutants, and inadequate vaccination coverage.12,13
Children, especially those under five years of age, are particularly susceptible due to their developing immune systems and frequent close contact with peers in school or daycare settings.14,15
Clinical Presentation
The clinical presentation of bacterial respiratory infections in children can vary depending on the pathogen involved and the site of infection. URTIs commonly manifest with symptoms such as: runny or stuffy nose, sore throat, mild cough, and low-grade fever.
Lower respiratory tract infections (LRTIs), such as bronchitis or pneumonia, often present with more severe symptoms, including: severe cough, chest pain, shortness of breath, high fever, fatigue, loss of appetite, and irritability. In some cases, children may also experience additional symptoms like headaches, body aches, and gastrointestinal disturbances.16
Diagnosis and Treatment
Accurate diagnosis of bacterial respiratory infections in children is crucial for appropriate treatment and prevention of complications. Healthcare providers typically consider the child’s medical history, physical examination findings, and laboratory tests to make a diagnosis. Laboratory tests may include: blood tests (e.g. complete blood count, C-reactive protein), sputum cultures, chest X-rays, rapid antigen detection tests and molecular diagnostic methods (e.g. PCR).17-19
The choice of diagnostic approach often depends on the severity of symptoms, the child’s age, and the suspected pathogen.3
Treatment of bacterial respiratory infections in children usually involves antibiotic therapy. The selection of antibiotics depends on the type of bacteria suspected or identified and its susceptibility to specific medications. Commonly prescribed antibiotics for pediatric respiratory infections include: amoxicillin, cephalosporins (e.g. cefuroxime, ceftriaxone, etc.), and macrolides (e.g. azithromycin, clarithromycin, etc). It is crucial to follow the prescribed dosage and complete the full course of antibiotics to ensure effective treatment and prevent the development of antibiotic resistance.20
Challenges in Pediatric Respiratory Infection Management
Despite advances in diagnostic techniques and treatment options, managing bacterial respiratory infections in children presents several challenges:
-
Differentiation between viral and bacterial infections: Many respiratory symptoms can be caused by both viruses and bacteria, making it difficult to determine the need for antibiotic treatment based on clinical presentation alone.5
-
Antibiotic resistance: The increasing prevalence of antibiotic-resistant bacteria complicates treatment decisions and emphasizes the need for accurate diagnostic methods to guide appropriate antibiotic use.4
-
Sample collection: Obtaining adequate samples for diagnostic testing can be challenging in young children, particularly for lower respiratory tract infections.7
-
Rapid diagnosis: The need for quick and accurate diagnosis is crucial in pediatric patients to initiate timely treatment and prevent complications.21
-
Overuse of antibiotics: Parental pressure and diagnostic uncertainty often lead to unnecessary antibiotic prescriptions, contributing to the growing problem of antibiotic resistance.14
Addressing these challenges requires a multifaceted approach, including improved diagnostic techniques, education of healthcare providers and parents, and the development of rapid, accurate POCT methods. The following sections will delve deeper into the various diagnostic approaches available for pediatric respiratory infections, with a focus on molecular detection methods and POCT.
Identification and Detection of Bacterial Respiratory Infections in Children
Accurate and timely identification of bacterial pathogens causing respiratory infections in children is crucial for effective treatment and management. This section explores various detection methods, ranging from conventional techniques to advanced molecular approaches (Table 1).22,23
Table 1.
Comparison of molecular, conventional and serological methods for detection of bacterial infections.
|
Method Characteristics
|
Molecular
|
Culture
|
Serological
|
Ref.
|
| Principle |
Amplification and detection |
Isolation and growth |
Detection of antibodies |
24,25
|
| Sensitivity |
High |
Variable |
Variable |
26-28
|
| Specificity |
High |
High |
Variable |
| Speed |
Rapid |
Can take days |
Rapid |
| Equipment |
PCR machine/Amplification kit |
Microbiology laboratory setup |
ELISA reader/Immunoassay device |
29-31
|
| Sample |
Minimal |
Large quantity |
Serum or other bodily fluids |
32-34
|
| Diagnostic purpose |
Identifying bacterial strains |
Identifying bacterial species |
Detecting past or current infection |
| Limitations |
Requires specialized equipment |
May miss slow-growing bacteria |
False positives/negatives possible |
| Examples of use |
PCR, Real-time PCR, LAMP, RPA, NASBA, etc. |
Blood agar plate culture |
ELISA, Western blot, etc. |
35-37
|
Conventional Detection Methods
Conventional methods have long been the cornerstone of bacterial identification in clinical settings. These methods include:
Culture-Based Techniques
Culturing remains a standard approach for identifying bacterial pathogens. However, it has limitations, particularly for fastidious organisms like Streptococcus pneumoniae. The sensitivity of culture tests can rapidly diminish after antibiotic administration, potentially leading to false-negative results.38 Despite its high specificity, blood culture positivity rates are typically less than 20% in pediatric patients with suspected bacterial infections.8
Gram Staining
This rapid and inexpensive method provides initial information about the morphology and gram-reaction of bacteria. However, it lacks specificity and cannot definitively identify the bacterial species.5
Biochemical Assays
These tests identify bacteria based on their metabolic characteristics. While useful, they can be time-consuming and may not differentiate between closely related species.39
Serological Methods
Serological testing, which detects specific antibodies, can be helpful when conventional methods fail to identify the cause of infection. However, these methods have limitations in patients with compromised immune systems and may not detect acute infections.40
Advanced Detection Methods
To address the limitations of conventional techniques, several advanced methods have been developed:
Molecular Detection Techniques
Nucleic acid amplification techniques, particularly PCR, have revolutionized the detection of respiratory pathogens. Multiplex real-time PCR is especially useful, offering rapid diagnosis with universal sensitivity and specificity of 75.9% and 96.5%, respectively.3 These methods can detect a very small number of organisms, whether viable or not, making them highly sensitive.41
Other molecular methods include:
-
Microarray technologies: These use two-dimensional microchips or three-dimensional beads for simultaneous detection of multiple pathogens.
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS): This technique rapidly identifies bacteria based on their protein profiles (Figure 1).2
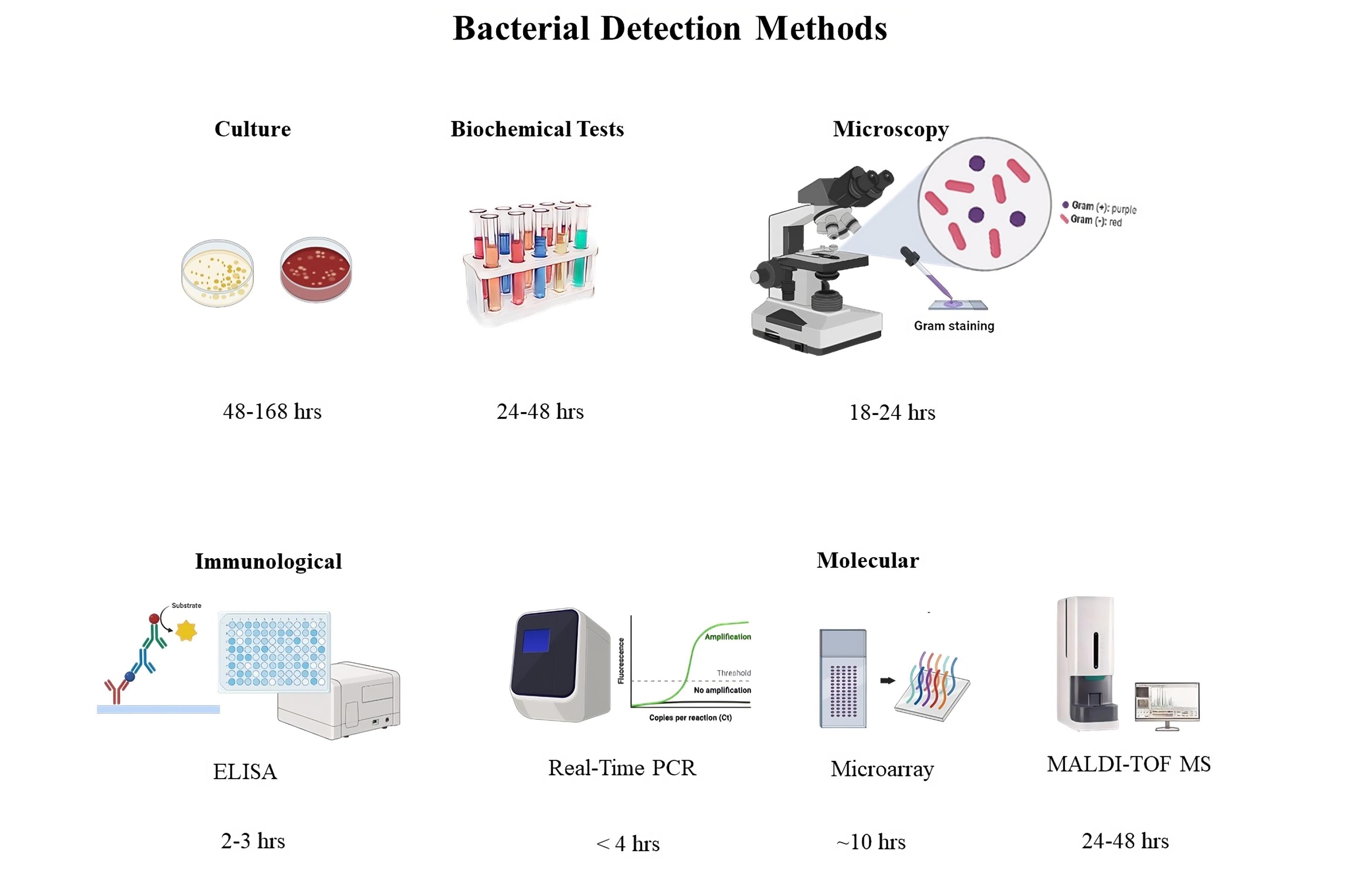
Figure 1.
Different Detection Methods Used for Bacterial Infections and their Turnaround Time
.
Different Detection Methods Used for Bacterial Infections and their Turnaround Time
Rapid Antigen Detection Tests
Immunochromatographic urinary antigen tests provide rapid diagnosis for certain pathogens like Streptococcus pneumoniae and Legionella pneumophila. However, their sensitivity and specificity can be lower in pediatric populations, particularly for pneumococcal infections.42
Point-of-Care Testing
POCT methods are designed to be easy to use, sensitive, and provide rapid results at or near the patient care site. These tests can be broadly categorized into two groups:
-
Tests determining the cause of infection.
-
Tests confirming the presence of specific pathogens.
For children older than eight years, urine tests for simultaneous identification of S. pneumoniae and L. pneumophila antigens are commonly used, with a sensitivity of 85%-89% (Figure 2).16
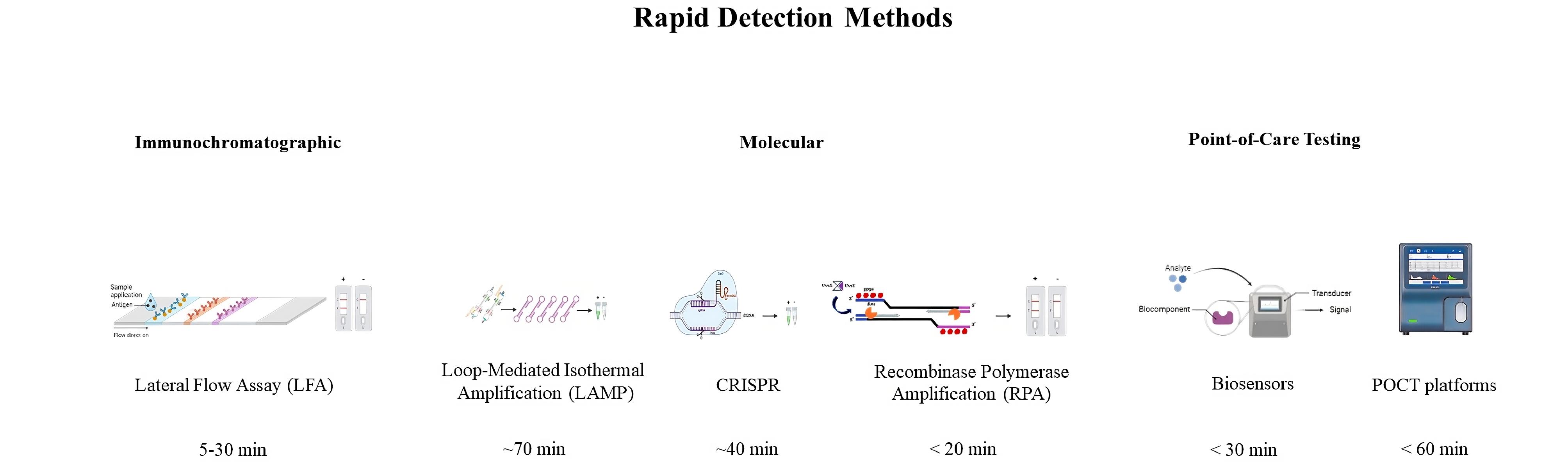
Figure 2.
Some Rapid Detection Methods used for Bacterial Infections and their Turnaround Time
.
Some Rapid Detection Methods used for Bacterial Infections and their Turnaround Time
Several PCR-based POCT platforms have been developed that can detect multiple respiratory pathogens within an hour. The Film Array respiratory panel, for instance, has shown promise in reducing hospitalization costs and intravenous antibiotic use.21
Other commercially available respiratory panels include QIAstat-Dx®, Unyvero, DendriChips®, and RespiFinder® SMART 22 FAST. These platforms offer rapid, multiplex detection of various respiratory pathogens.16
Emerging Biomarkers
Research is ongoing into novel biomarkers for respiratory infections. The C-reactive protein (CRP) test and procalcitonin concentration measurements show promise as POCT tests, although their utility in pediatric populations requires further investigation.43
Other potential biomarkers include:
-
VAPrapid-2: This test measures interleukins IL-1β and IL-8.44,45
-
HostDx Sepsis test: This assay examines a large battery of host transcriptome markers in blood for pneumonia diagnosis.43
These emerging biomarkers may offer new avenues for rapid and accurate diagnosis of respiratory infections in children, but further clinical trials are needed to validate their effectiveness.43
Challenges and Future Directions
Despite significant advancements in detection methods, several challenges remain:
-
Sample collection: Obtaining adequate samples, particularly from young children, can be difficult.46,47
-
Interpretation of results: The presence of bacterial DNA does not necessarily indicate an active infection, potentially leading to over-diagnosis.48,49
-
Cost and accessibility: Advanced molecular techniques may not be readily available in all healthcare settings, particularly in resource-limited areas.50,51
-
Rapid detection of antibiotic resistance: There is an ongoing need for methods that can quickly identify antibiotic-resistant strains to guide appropriate treatment.52
Future research should focus on developing more accessible, rapid, and comprehensive diagnostic tools that can simultaneously detect pathogens and their antibiotic resistance profiles. Integration of artificial intelligence and machine learning algorithms with diagnostic platforms may further enhance the accuracy and speed of diagnosis.53,54
Antibiotic Resistance in Pediatric Respiratory Infections
Antibiotic resistance is a growing concern in the management of pediatric respiratory infections. The overuse and misuse of antibiotics have led to the emergence of resistant bacterial strains, complicating treatment and potentially leading to more severe outcomes. This section explores the challenges posed by antibiotic resistance and the methods used to detect resistant strains in pediatric respiratory infections.55,56
Prevalence and Impact of Antibiotic Resistance
The prevalence of antibiotic-resistant bacteria in pediatric respiratory infections has been increasing globally. A study by Torres et al found that more than 70% of uncertain diagnoses and prescriptions of antibiotics contribute to the rising antibacterial resistance in children.4 This trend is particularly concerning for common respiratory pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus.
The impact of antibiotic resistance in pediatric populations includes: increased morbidity and mortality, prolonged hospital stays, higher healthcare costs, limited treatment options, and potential for spread of resistant strains in communities.57,58
Factors Contributing to Antibiotic Resistance
Several factors contribute to the development and spread of antibiotic resistance in pediatric respiratory infections:
-
Overuse of antibiotics: Unnecessary prescription of antibiotics for viral infections or minor bacterial infections that could resolve without treatment.9
-
Inappropriate antibiotic selection: Use of broad-spectrum antibiotics when narrow-spectrum drugs would suffice.20
-
Incomplete treatment courses: Failure to complete the full course of prescribed antibiotics, allowing resistant bacteria to survive and multiply.16
-
Antibiotic use in agriculture: The use of antibiotics in livestock can lead to the development of resistant bacteria that may be transmitted to humans.2
-
Poor infection control practices: Inadequate hygiene and infection control measures in healthcare settings can facilitate the spread of resistant bacteria.3
Methods to Identify Bacterial Resistance
Rapid and accurate detection of antibiotic-resistant bacteria is crucial for effective treatment of pediatric respiratory infections. Various methods have been developed to identify resistant strains:
Phenotypic Methods
Traditional phenotypic methods involve culturing bacteria in the presence of antibiotics to determine their susceptibility. These methods include:
-
Disk diffusion test: Measures the zone of inhibition around antibiotic-impregnated disks on agar plates.59
-
Broth dilution: Determines the minimum inhibitory concentration (MIC) of antibiotics required to inhibit bacterial growth.60
-
Automated systems: Such as VITEK and Phoenix systems, which provide rapid antibiotic susceptibility results.5,59
While these methods are widely used, they typically require 24-48 hours to obtain results, which can delay appropriate treatment.61
Molecular Methods
Molecular techniques offer faster and more specific detection of antibiotic resistance genes. These methods include:
-
PCR-based assays: Detecting specific resistance genes, such as mecA for methicillin resistance in Staphylococcus aureus.
-
Microarray technologies: Allowing simultaneous detection of multiple resistance genes.
-
Whole genome sequencing: Provides comprehensive information about resistance genes and other genetic factors contributing to antibiotic resistance.4
Rapid Diagnostic Platforms
Several rapid diagnostic platforms have been developed to detect antibiotic-resistant bacteria in clinical samples:
-
Verigene® system: This platform can detect resistance genes such as mecA and vanA /B in gram-positive bacteria, and genes encoding extended-spectrum beta-lactamases (ESBLs) and carbapenemases in gram-negative bacteria.6,7
-
FilmArray Blood Culture Identification Panel: This nested PCR-based system can detect resistance genes including mecA, vanA /B, and KPC.5
-
GeneXpert® system: Offers cartridges for rapid detection of MRSA in various clinical samples.8
-
Other platforms: Including NucliSENS EasyQ® KPC, Xpert® Carba-R, eazyplex® SuperBug CRE, and Check-Direct CPE, which target specific resistance mechanisms.39,41
Emerging Technologies
New approaches for detecting antibiotic resistance are continually being developed:
-
CRISPR-Cas-based diagnostics: These methods exploit the specificity of CRISPR-Cas systems to detect antibiotic resistance genes rapidly and accurately.35
-
Nanopore sequencing: This technology allows real-time sequencing of bacterial genomes, potentially providing comprehensive resistance profiles within hours.34
-
Machine learning algorithms: These can predict antibiotic resistance based on genomic data, potentially accelerating the identification of novel resistance mechanisms.32
Challenges and Future Directions
Despite advances in detection methods, several challenges persist in addressing antibiotic resistance in pediatric respiratory infections:
-
Cost and accessibility: Many advanced detection methods are expensive and not widely available, particularly in resource-limited settings.
-
Turnaround time: While molecular methods are faster than traditional culture-based approaches, there is still a need for even more rapid diagnostics to guide immediate treatment decisions.
-
Complexity of resistance mechanisms: The continual evolution of resistance mechanisms necessitates ongoing updates to detection methods.
-
Interpretation of results: The presence of resistance genes does not always correlate with phenotypic resistance, complicating clinical decision-making.62,63
Future research should focus on developing more accessible, rapid, and comprehensive resistance detection methods. Integration of resistance testing into point-of-care devices could significantly improve the management of pediatric respiratory infections. Additionally, efforts to promote antimicrobial stewardship and judicious use of antibiotics in pediatric populations are crucial to mitigate the spread of antibiotic resistance.22,43
Point-of-Care Testing for Pediatric Respiratory Infections
POCT has emerged as a promising approach for rapid diagnosis of pediatric respiratory infections. These tests offer the potential for faster, more accurate diagnoses, leading to improved patient management and more judicious use of antibiotics. This section explores the various POCT methods available for pediatric respiratory infections, their advantages and limitations, and their potential impact on patient care.43,64
Overview of POCT in Pediatric Respiratory Infections
POCT refers to diagnostic testing performed at or near the site of patient care. These tests are designed to be user-friendly, provide rapid results, and facilitate immediate clinical decision-making. In the context of pediatric respiratory infections, POCT can be broadly categorized into two main groups: (1) Tests that determine the cause of infection. (2) Tests that confirm the presence of specific pathogens.
The adoption of POCT in pediatric care has been driven by several factors, including the need for rapid diagnosis, the desire to reduce unnecessary antibiotic use, and the goal of improving overall patient outcomes.16
Types of POCT for Pediatric Respiratory Infections
Rapid Antigen Detection Tests
These tests detect specific antigens from respiratory pathogens in patient samples. Common examples include:
-
Rapid Streptococcus A test: Detects group A streptococcal antigen in throat swabs.
-
Influenza rapid antigen tests: Detect influenza A and B viral antigens in nasopharyngeal samples.
-
RSV rapid antigen tests: Identify respiratory syncytial virus antigens in nasal secretions.
While these tests offer rapid results, their sensitivity can be variable, and negative results often require confirmation with more sensitive methods.21
Molecular POCT Platforms
Molecular POCT platforms use nucleic acid amplification techniques to detect pathogens. These methods offer higher sensitivity and specificity compared to antigen-based tests.65 Examples include:
-
FilmArray Respiratory Panel (BioFire Diagnostics): This multiplex PCR system can detect multiple respiratory pathogens simultaneously within about an hour. Studies have shown that its use can reduce hospitalization costs and intravenous antibiotic use in pediatric patients.21
-
Xpert Xpress (Cepheid): Offers rapid PCR-based detection of influenza and RSV, with results available in less than 30 minutes.66
-
ID NOW (Abbott): Provides molecular detection of influenza A & B, RSV, and SARS-CoV-2 in 13 minutes or less.67
-
COBAS Liat (Roche): Offers PCR-based detection of multiple respiratory pathogens with a turnaround time of 20 minutes.68
These molecular POCT platforms have shown high sensitivity and specificity in pediatric populations, potentially improving diagnostic accuracy and reducing time to appropriate treatment.69
Isothermal molecular tests to identify bacterial resistance
The detection and diagnosis of bacterial infections play a crucial role in effective disease management and prevention. Traditional detection methods often involve complex, time-consuming, and expensive processes, hindering their suitability for rapid diagnostics in resource-limited settings. In recent years, isothermal methods have emerged as promising alternatives due to their simplicity, cost-effectiveness, and capability for POCT. This article aims to provide an overview of isothermal methods utilized in the detection of bacterial infections and their potential applications.9,70,71
A. Loop-Mediated Isothermal Amplification
Loop-mediated isothermal amplification (LAMP) is a widely recognized method for the detection of various bacterial infections. By utilizing multiple primers, LAMP allows for rapid amplification of target DNA under isothermal conditions. LAMP offers high specificity, sensitivity, and simplicity, making it suitable for point-of-care diagnostics. For instance, LAMP has been successfully employed for the detection of pathogens such as Staphylococcus aureus, Salmonella spp., and Mycobacterium tuberculosis.9,16
B. Recombinase Polymerase Amplification
Recombinase polymerase amplification (RPA) is another isothermal technique gaining popularity for bacterial infection detection.72 RPA utilizes recombinase enzymes and DNA polymerases to amplify target DNA at a constant temperature. RPA is highly specific, fast, and portable, with applications ranging from environmental monitoring to clinical diagnostics. Studies have demonstrated successful utilization of RPA in detecting bacterial pathogens, including Escherichia coli and Klebsiella pneumoniae.16,73
C. Nucleic Acid Sequence-Based Amplification
Nucleic acid sequence-based amplification (NASBA) is an isothermal amplification technique that enables the detection of RNA-based infections.74 NASBA utilizes reverse transcriptase and RNA polymerase to amplify target RNA at a constant temperature. This method offers high specificity and sensitivity, making it suitable for detecting bacterial infections caused by RNA viruses. Notably, NASBA has been employed in the detection of bacterial pathogens such as Helicobacter pylori and Chlamydia trachomatis.3,74
D. Recombinase-Aided Amplification
Recombinase-aided amplification (RAA) is an isothermal nucleic acid amplification method that relies on recombinase enzymes and DNA polymerases. RAA provides rapid amplification of target DNA or RNA, making it suitable for point-of-care diagnostics. With its simplicity and robustness, RAA shows great potential for detecting bacterial pathogens, including MRSA and Mycobacterium tuberculosis.16,75
E. Whole Genome Amplification
Whole genome amplification (WGA) is an isothermal technique used to amplify the entire genome of a bacterial pathogen. By amplifying genomic DNA templates, WGA facilitates downstream molecular analysis and characterization of bacterial infections.76 WGA has been applied to the detection of various bacteria, facilitating genomic research and surveillance efforts.4,77 Isothermal methods have revolutionized the detection and diagnosis of bacterial infections due to their simplicity, cost-effectiveness, and suitability for POCT. These methods offer rapid, sensitive, and specific results, making them valuable tools for healthcare professionals, researchers, and diagnostic laboratories. As technology advances, isothermal methods hold the potential to further enhance early detection, surveillance, and management strategies for bacterial infections.78,79
Microfluidic Devices
Emerging microfluidic technologies are enabling the development of novel POCT devices for respiratory infections. These devices can integrate sample preparation, amplification, and detection into a single, compact system. While many of these technologies are still in development, they show promise for rapid, sensitive, and specific detection of respiratory pathogens in pediatric patients.80
Host Response Biomarker Tests
Some POCT methods focus on detecting host response biomarkers to differentiate between viral and bacterial infections. Examples include:
-
CRP POCT: Measures levels of CRP, an acute-phase protein that increases in response to inflammation.81,82
-
Procalcitonin POCT: Detects levels of procalcitonin, which tends to be elevated in bacterial infections.83,84
While these tests can provide valuable information, their interpretation in pediatric populations can be challenging and often requires consideration alongside other clinical and laboratory findings.43
Advantages of POCT in Pediatric Respiratory Infections
Implementation of POCT in managing pediatric respiratory infections offers several potential benefits:
-
Rapid results: POCT can provide results within minutes to hours, allowing for faster clinical decision-making.85,86
-
Improved antibiotic stewardship: Rapid identification of viral pathogens can reduce unnecessary antibiotic prescriptions.87,88
-
Enhanced patient care: Faster diagnosis can lead to more timely and appropriate treatment.85,89
-
Reduced healthcare costs: POCT may decrease the need for additional testing and shorten hospital stays.89,90
-
Improved infection control: Rapid identification of pathogens can facilitate timely implementation of infection control measures.91,92
Limitations and Challenges of POCT
Despite its potential benefits, POCT for pediatric respiratory infections faces several challenges;43,64,89
-
Cost: Some POCT platforms, particularly molecular-based systems, can be expensive to implement and maintain.93,94
-
Quality control: Ensuring consistent test performance across different operators and settings can be challenging.95,96
-
Result interpretation: Healthcare providers need proper training to interpret POCT results in the context of clinical presentation.86,92,97
-
Limited test menu: Some POCT platforms may not cover all pathogens of interest.98,99
-
Regulatory considerations: POCT devices must meet regulatory requirements, which can vary by region.92,100
Impact on Patient Care and Antibiotic Stewardship
The implementation of POCT in pediatric respiratory infections has shown promising results in improving patient care and promoting antibiotic stewardship. A randomized clinical trial by Mattila et al found that the use of POCT for respiratory pathogens significantly reduced antibiotic use in children without compromising patient outcomes.16
Moreover, rapid identification of viral pathogens through POCT can help reassure parents and clinicians, potentially reducing unnecessary antibiotic prescriptions and follow-up visits. This approach aligns with global efforts to combat antibiotic resistance by promoting more judicious use of antimicrobial agents in pediatric populations.9
Future Directions
The field of POCT for pediatric respiratory infections continues to evolve rapidly.64,101 Future developments may include:
-
Integration of artificial intelligence: Machine learning algorithms could enhance the interpretation of POCT results, potentially improving diagnostic accuracy.102,103
-
Multiplexed detection of pathogens and antibiotic resistance: Next-generation POCT devices may simultaneously identify pathogens and their antibiotic resistance profiles.53,54
-
Wearable and smartphone-connected devices: These could enable continuous monitoring of respiratory parameters and facilitate remote diagnosis.104,105
-
Improved sample collection methods: Development of less invasive and more child-friendly sampling techniques could enhance the acceptability of POCT in pediatric populations.106,107
Conclusion
Molecular testing has significantly improved respiratory pathogen detection and is now regarded as the new “gold standard.” Although these tests have grown in popularity, criteria such as patient demographics (adult, pediatric, and immunocompromised), laboratory size, testing purpose (regular or urgent care), and cost-benefit ratio should be addressed before implementing a specific assay. Molecular diagnostics offer high sensitivity and the potential for timely delivery of actionable information, which can reduce diagnostic uncertainty and help inform early treatment decisions more effectively than conventional culture and antigen-based methods. Decisions for the deployment of molecular diagnostics in the hospital laboratory and for the hospital system should take into account their use in guiding procedures and policies, such as in-hospital epidemiology and antibiotic stewardship. As technology advances and data-supporting best practices emerge, policies for successful utilization must be assessed on a constant basis. Future research will be required to demonstrate the clinical efficacy of current and future tests in a variety of patient demographics and resource-constrained situations.
Acknowledgements
We would like to extend our sincere appreciation to Zahra Tajari (Dep. of Biology, Gorgan Branch, Islamic Azad University) and Morteza Banakar (Dental Research Center, Dentistry Research Institute, Tehran University of Medical Sciences) for their valuable assistance and guidance.
Competing Interests
None of the authors have a conflict of interest to disclose.
Ethical Approval
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Álvarez-Ojeda MG, Saldaña-Fuentes C, Ballesteros-Elizondo MR, Martínez-Vázquez IO, López-Merino A, Briones Lara E, et al. [Comparison of the tests polymerase chain reaction, serology, and blood culture with respect to sensitivity and specificity for detection of Brucella spp in human samples]. Gac Med Mex 2015;151(5):620-7. [Spanish].
- Boyles TH, Wasserman S. Diagnosis of bacterial infection. S Afr Med J 2015; 105(5):419. doi: 10.7196/samj.9647 [Crossref] [ Google Scholar]
- Rytter H, Jamet A, Coureuil M, Charbit A, Ramond E. Which current and novel diagnostic avenues for bacterial respiratory diseases?. Front Microbiol 2020; 11:616971. doi: 10.3389/fmicb.2020.616971 [Crossref] [ Google Scholar]
- Torres A, Menéndez R, España PP, Fernández-Villar JA, Marimón JM, Cilloniz C. The evolution and distribution of pneumococcal serotypes in adults hospitalized with community-acquired pneumonia in Spain using a serotype-specific urinary antigen detection test: the CAPA study, 2011-2018. Clin Infect Dis 2021; 73(6):1075-85. doi: 10.1093/cid/ciab307 [Crossref] [ Google Scholar]
- Noviello S, Huang DB. The basics and the advancements in diagnosis of bacterial lower respiratory tract infections. Diagnostics (Basel) 2019; 9(2):37. doi: 10.3390/diagnostics9020037 [Crossref] [ Google Scholar]
- Senchyna F, Gaur R, Sandlund J, Truong C, Tremintin G, Küeltz D, et al. Diverse mechanisms of resistance in carbapenem-resistant Enterobacteriaceae at a health care system in Silicon Valley, California. bioRxiv [Preprint]. April 10, 2018. Available from: https://www.biorxiv.org/content/10.1101/298513v1.
- Senchyna F, Gaur RL, Sandlund J, Truong C, Tremintin G, Kültz D. Diversity of resistance mechanisms in carbapenem-resistant Enterobacteriaceae at a health care system in Northern California, from 2013 to 2016. Diagn Microbiol Infect Dis 2019; 93(3):250-7. doi: 10.1016/j.diagmicrobio.2018.10.004 [Crossref] [ Google Scholar]
- Mattila JT, Fine MJ, Limper AH, Murray PR, Chen BB, Lin PL. Pneumonia Treatment and diagnosis. Ann Am Thorac Soc 2014; 11(Suppl 4):S189-92. doi: 10.1513/AnnalsATS.201401-027PL [Crossref] [ Google Scholar]
- Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014; 5(6):229-41. doi: 10.1177/2042098614554919 [Crossref] [ Google Scholar]
- Claassen-Weitz S, Lim KYL, Mullally C, Zar HJ, Nicol MP. The association between bacteria colonizing the upper respiratory tract and lower respiratory tract infection in young children: a systematic review and meta-analysis. Clin Microbiol Infect 2021; 27(9):1262-70. doi: 10.1016/j.cmi.2021.05.034 [Crossref] [ Google Scholar]
- Diaz-Diaz A, Bunsow E, Garcia-Maurino C, Moore-Clingenpeel M, Naples J, Juergensen A. Nasopharyngeal codetection of Haemophilus influenzae and Streptococcus pneumoniae shapes respiratory syncytial virus disease outcomes in children. J Infect Dis 2022; 225(5):912-23. doi: 10.1093/infdis/jiab481 [Crossref] [ Google Scholar]
- Zhang N, Chen W, Chan PT, Yen HL, Tang JW, Li Y. Close contact behavior in indoor environment and transmission of respiratory infection. Indoor Air 2020; 30(4):645-61. doi: 10.1111/ina.12673 [Crossref] [ Google Scholar]
- Singh NK, Kumar N, Singh AK. Physiology to disease transmission of respiratory tract infection: a narrative review. Infect Disord Drug Targets 2021; 21(6):e170721188930. doi: 10.2174/1871526520666201209145908 [Crossref] [ Google Scholar]
- Abu Hammour K, Al-Saleh S, Abu Hammour W. Parental views of antibiotic use in children with upper respiratory tract infections in Dubai. Eur J Integr Med 2019; 29:100917. doi: 10.1016/j.eujim.2019.05.003 [Crossref] [ Google Scholar]
- Kurt G, Serdaroğlu HU. Prevalence of infectious diseases in children at preschool education institutions and stakeholder opinions. Children (Basel) 2024; 11(4):447. doi: 10.3390/children11040447 [Crossref] [ Google Scholar]
- Mattila S, Paalanne N, Honkila M, Pokka T, Tapiainen T. Effect of point-of-care testing for respiratory pathogens on antibiotic use in children: a randomized clinical trial. JAMA Netw Open 2022; 5(6):e2216162. doi: 10.1001/jamanetworkopen.2022.16162 [Crossref] [ Google Scholar]
- Zhao L, Wu L, Xu W, Wei J, Niu X, Liu G. Diagnostic techniques for critical respiratory infections: update on current methods. Heliyon 2023; 9(8):e18957. doi: 10.1016/j.heliyon.2023.e18957 [Crossref] [ Google Scholar]
- Bhowmik A. Role of diagnostic procedures in managing human bacterial infections: a comprehensive overview. Arch Hematol Case Rep Rev 2023; 8(1):8-19. doi: 10.17352/ahcrr.000043 [Crossref] [ Google Scholar]
- Gao CA, Huston JC, Toro PV, Gautam S, Dela Cruz CS. Molecular diagnostics in pulmonary infections. In: Gomez JL, Himes BE, Kaminski N, eds. Precision in Pulmonary, Critical Care, and Sleep Medicine: A Clinical and Research Guide. Cham: Springer; 2020. p. 167-84. 10.1007/978-3-030-31507-8_12.
- Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis 2011; 52 Suppl 4:S296-304. doi: 10.1093/cid/cir045 [Crossref] [ Google Scholar]
- Shen N, Zhou Y, Zhou Y, Luo L, Chen W, Wang J. Evaluation of molecular point-of-care testing for respiratory pathogens in children with respiratory infections: a retrospective case-control study. Front Cell Infect Microbiol 2021; 11:778808. doi: 10.3389/fcimb.2021.778808 [Crossref] [ Google Scholar]
- Ciccone EJ, Kabugho L, Baguma E, Muhindo R, Juliano JJ, Mulogo E. Rapid diagnostic tests to guide case management of and improve antibiotic stewardship for pediatric acute respiratory illnesses in resource-constrained settings: a prospective cohort study in Southwestern Uganda. Microbiol Spectr 2021; 9(3):e0169421. doi: 10.1128/Spectrum.01694-21 [Crossref] [ Google Scholar]
- Lin CY, Hwang D, Chiu NC, Weng LC, Liu HF, Mu JJ. Increased detection of viruses in children with respiratory tract infection using PCR. Int J Environ Res Public Health 2020; 17(2):564. doi: 10.3390/ijerph17020564 [Crossref] [ Google Scholar]
- Fiedoruk K, Daniluk T, Rozkiewicz D, Zaremba ML, Oldak E, Sciepuk M. Conventional and molecular methods in the diagnosis of community-acquired diarrhoea in children under 5 years of age from the north-eastern region of Poland. Int J Infect Dis 2015; 37:145-51. doi: 10.1016/j.ijid.2015.06.028 [Crossref] [ Google Scholar]
- Rhoads DD, Wolcott RD, Sun Y, Dowd SE. Comparison of culture and molecular identification of bacteria in chronic wounds. Int J Mol Sci 2012; 13(3):2535-50. doi: 10.3390/ijms13032535 [Crossref] [ Google Scholar]
- Herrera M, Aguilar YA, Rueda ZV, Muskus C, Vélez LA. Comparison of serological methods with PCR-based methods for the diagnosis of community-acquired pneumonia caused by atypical bacteria. J Negat Results Biomed 2016; 15:3. doi: 10.1186/s12952-016-0047-y [Crossref] [ Google Scholar]
- Fenollar F, Raoult D. Molecular diagnosis of bloodstream infections caused by non-cultivable bacteria. Int J Antimicrob Agents 2007; 30 Suppl 1:S7-15. doi: 10.1016/j.ijantimicag.2007.06.024 [Crossref] [ Google Scholar]
- Opota O, Jaton K, Greub G. Microbial diagnosis of bloodstream infection: towards molecular diagnosis directly from blood. Clin Microbiol Infect 2015; 21(4):323-31. doi: 10.1016/j.cmi.2015.02.005 [Crossref] [ Google Scholar]
- Galluzzi L, Magnani M, Saunders N, Harms C, Bruce IJ. Current molecular techniques for the detection of microbial pathogens. Sci Prog 2007; 90(Pt 1):29-50. doi: 10.3184/003685007780440521 [Crossref] [ Google Scholar]
- Greub G, Sahli R, Brouillet R, Jaton K. Ten years of R&D and full automation in molecular diagnosis. Future Microbiol 2016; 11(3):403-25. doi: 10.2217/fmb.15.152 [Crossref] [ Google Scholar]
- Reta DH, Tessema TS, Ashenef AS, Desta AF, Labisso WL, Gizaw ST. Molecular and immunological diagnostic techniques of medical viruses. Int J Microbiol 2020; 2020:8832728. doi: 10.1155/2020/8832728 [Crossref] [ Google Scholar]
- Hameed S, Xie L, Ying Y. Conventional and emerging detection techniques for pathogenic bacteria in food science: a review. Trends Food Sci Technol 2018; 81:61-73. doi: 10.1016/j.tifs.2018.05.020 [Crossref] [ Google Scholar]
- Law JW, Ab Mutalib NS, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol 2014; 5:770. doi: 10.3389/fmicb.2014.00770 [Crossref] [ Google Scholar]
- Váradi L, Luo JL, Hibbs DE, Perry JD, Anderson RJ, Orenga S. Methods for the detection and identification of pathogenic bacteria: past, present, and future. Chem Soc Rev 2017; 46(16):4818-32. doi: 10.1039/c6cs00693k [Crossref] [ Google Scholar]
- Deusenbery C, Wang Y, Shukla A. Recent innovations in bacterial infection detection and treatment. ACS Infect Dis 2021; 7(4):695-720. doi: 10.1021/acsinfecdis.0c00890 [Crossref] [ Google Scholar]
- Maurin M. Francisellatularensis, tularemia and serological diagnosis. Front Cell Infect Microbiol 2020; 10:512090. doi: 10.3389/fcimb.2020.512090 [Crossref] [ Google Scholar]
- Coleman JS, Gaydos CA. Molecular diagnosis of bacterial vaginosis: an update. J Clin Microbiol 2018; 56(9):e00342-18. doi: 10.1128/jcm.00342-18 [Crossref] [ Google Scholar]
- Hasanuzzaman M, Saha S, Malaker R, Rahman H, Sajib MS, Das RC. Comparison of culture, antigen test, and polymerase chain reaction for pneumococcal detection in cerebrospinal fluid of children. J Infect Dis 2021; 224(12 Suppl 2):S209-17. doi: 10.1093/infdis/jiab073 [Crossref] [ Google Scholar]
- Lung M, Codina G. Molecular diagnosis in HAP/VAP. Curr Opin Crit Care 2012; 18(5):487-94. doi: 10.1097/MCC.0b013e3283577d37 [Crossref] [ Google Scholar]
- Saenghirunvattana S, Suwangool P, Ortiz PB, Noomcharoen C, Wongkhot N. Rapid detection of respiratory pathogen 33 in cases of acute respiratory tract infection. Bangkok Med J 2020; 16(1):9. doi: 10.31524/bkkmedj.2020.16.1.002 [Crossref] [ Google Scholar]
- Ryan U, Zahedi A, Paparini A. Cryptosporidium in humans and animals-a one health approach to prophylaxis. Parasite Immunol 2016; 38(9):535-47. doi: 10.1111/pim.12350 [Crossref] [ Google Scholar]
- Couturier MR, Graf EH, Griffin AT. Urine antigen tests for the diagnosis of respiratory infections: legionellosis, histoplasmosis, pneumococcal pneumonia. Clin Lab Med 2014; 34(2):219-36. doi: 10.1016/j.cll.2014.02.002 [Crossref] [ Google Scholar]
- Dhesi Z, Enne VI, O’Grady J, Gant V, Livermore DM. Rapid and point-of-care testing in respiratory tract infections: an antibiotic guardian?. ACS Pharmacol Transl Sci 2020; 3(3):401-17. doi: 10.1021/acsptsci.0c00027 [Crossref] [ Google Scholar]
- Póvoa P, Coelho L. Which biomarkers can be used as diagnostic tools for infection in suspected sepsis?. Semin Respir Crit Care Med 2021; 42(5):662-71. doi: 10.1055/s-0041-1735148 [Crossref] [ Google Scholar]
- Hellyer TP, McAuley DF, Walsh TS, Anderson N, Conway Morris A, Singh S. Biomarker-guided antibiotic stewardship in suspected ventilator-associated pneumonia (VAPrapid2): a randomised controlled trial and process evaluation. Lancet Respir Med 2020; 8(2):182-91. doi: 10.1016/s2213-2600(19)30367-4 [Crossref] [ Google Scholar]
- Tribolet L, Kerr E, Cowled C, Bean AG, Stewart CR, Dearnley M. MicroRNA biomarkers for infectious diseases: from basic research to biosensing. Front Microbiol 2020; 11:1197. doi: 10.3389/fmicb.2020.01197 [Crossref] [ Google Scholar]
- Zandstra J, Jongerius I, Kuijpers TW. Future biomarkers for infection and inflammation in febrile children. Front Immunol 2021; 12:631308. doi: 10.3389/fimmu.2021.631308 [Crossref] [ Google Scholar]
- Lee HG, Choi W, Yang SY, Kim DH, Park SG, Lee MY. PCR-coupled paper-based surface-enhanced Raman scattering (SERS) sensor for rapid and sensitive detection of respiratory bacterial DNA. Sens Actuators B Chem 2021; 326:128802. doi: 10.1016/j.snb.2020.128802 [Crossref] [ Google Scholar]
- Mbelele PM, Sabiiti W, Heysell SK, Sauli E, Mpolya EA, Mfinanga S. Use of a molecular bacterial load assay to distinguish between active TB and post-TB lung disease. Int J Tuberc Lung Dis 2022; 26(3):276-8. doi: 10.5588/ijtld.21.0459 [Crossref] [ Google Scholar]
- Mitsakakis K, Kaman WE, Elshout G, Specht M, Hays JP. Challenges in identifying antibiotic resistance targets for point-of-care diagnostics in general practice. Future Microbiol 2018; 13(10):1157-64. doi: 10.2217/fmb-2018-0084 [Crossref] [ Google Scholar]
- Singh S, Numan A, Cinti S. Point-of-care for evaluating antimicrobial resistance through the adoption of functional materials. Anal Chem 2022; 94(1):26-40. doi: 10.1021/acs.analchem.1c03856 [Crossref] [ Google Scholar]
- Dietvorst J, Vilaplana L, Uria N, Marco MP, Muñoz-Berbel X. Current and near-future technologies for antibiotic susceptibility testing and resistant bacteria detection. Trends Analyt Chem 2020; 127:115891. doi: 10.1016/j.trac.2020.115891 [Crossref] [ Google Scholar]
- Kaprou GD, Bergšpica I, Alexa EA, Alvarez-Ordóñez A, Prieto M. Rapid methods for antimicrobial resistance diagnostics. Antibiotics (Basel) 2021; 10(2):209. doi: 10.3390/antibiotics10020209 [Crossref] [ Google Scholar]
- Yamin D, Uskoković V, Wakil AM, Goni MD, Shamsuddin SH, Mustafa FH. Current and future technologies for the detection of antibiotic-resistant bacteria. Diagnostics (Basel) 2023; 13(20):3246. doi: 10.3390/diagnostics13203246 [Crossref] [ Google Scholar]
- Medernach RL, Logan LK. The growing threat of antibiotic resistance in children. Infect Dis Clin North Am 2018; 32(1):1-17. doi: 10.1016/j.idc.2017.11.001 [Crossref] [ Google Scholar]
- Hersh AL, Jackson MA, Hicks LA. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics 2013; 132(6):1146-54. doi: 10.1542/peds.2013-3260 [Crossref] [ Google Scholar]
- Romandini A, Pani A, Schenardi PA, Pattarino GAC, De Giacomo C, Scaglione F. Antibiotic resistance in pediatric infections: global emerging threats, predicting the near future. Antibiotics (Basel) 2021; 10(4):393. doi: 10.3390/antibiotics10040393 [Crossref] [ Google Scholar]
- Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 2006; 42 Suppl 2:S82-9. doi: 10.1086/499406 [Crossref] [ Google Scholar]
- Bonnin RA, Emeraud C, Jousset AB, Naas T, Dortet L. Comparison of disk diffusion, MIC test strip and broth microdilution methods for cefiderocol susceptibility testing on carbapenem-resistant enterobacterales. Clin Microbiol Infect 2022;28(8):1156.e1-1156.e5. 10.1016/j.cmi.2022.04.013.
- Morris CP, Bergman Y, Tekle T, Fissel JA, Tamma PD, Simner PJ. Cefiderocol antimicrobial susceptibility testing against multidrug-resistant gram-negative bacilli: a comparison of disk diffusion to broth microdilution. J Clin Microbiol 2020; 59(1):e01649-20. doi: 10.1128/jcm.01649-20 [Crossref] [ Google Scholar]
- Yiş R. Comparison of broth microdilution method with BD phoenix, micro scan and e-test for carbapenem-resistant Enterobacterales colistin susceptibility testing. J Acad Res Med 2022; 12(2):61-5. doi: 10.4274/jarem.galenos.2022.70299 [Crossref] [ Google Scholar]
- Yee R, Dien Bard J, Simner PJ. The genotype-to-phenotype dilemma: how should laboratories approach discordant susceptibility results?. J Clin Microbiol 2021; 59(6):e00138-20. doi: 10.1128/jcm.00138-20 [Crossref] [ Google Scholar]
- Hassoun-Kheir N, Stabholz Y, Kreft JU, de la Cruz R, Romalde JL, Nesme J. Comparison of antibiotic-resistant bacteria and antibiotic resistance genes abundance in hospital and community wastewater: a systematic review. Sci Total Environ 2020; 743:140804. doi: 10.1016/j.scitotenv.2020.140804 [Crossref] [ Google Scholar]
- Zhang Z, Ma P, Ahmed R, Wang J, Akin D, Soto F. Advanced point-of-care testing technologies for human acute respiratory virus detection. Adv Mater 2022; 34(1):e2103646. doi: 10.1002/adma.202103646 [Crossref] [ Google Scholar]
- Suea-Ngam A, Bezinge L, Mateescu B, Howes PD, de Mello AJ, Richards DA. Enzyme-assisted nucleic acid detection for infectious disease diagnostics: moving toward the point-of-care. ACS Sens 2020; 5(9):2701-23. doi: 10.1021/acssensors.0c01488 [Crossref] [ Google Scholar]
- Mboumba Bouassa RS, Tonen-Wolyec S, Rodary J, Bélec L. Comparative practicability and analytical performances of Credo VitaPCRTM Flu A&B and Cepheid Xpert® Xpress Flu/RSV platforms. Diagn Microbiol Infect Dis 2021; 100(4):115381. doi: 10.1016/j.diagmicrobio.2021.115381 [Crossref] [ Google Scholar]
- Bottino P, Massarino C, Leli C, Scomparin E, Bara C, Gotta F. Evaluation of a commercial rapid molecular point-of-care assay for differential diagnosis between SARS-CoV-2 and Flu A/B infections in a pediatric setting. Viruses 2024; 16(10):1638. doi: 10.3390/v16101638 [Crossref] [ Google Scholar]
- Hansen G, Marino J, Wang ZX, Beavis KG, Rodrigo J, Labog K. Clinical performance of the point-of-care cobas Liat for detection of SARS-CoV-2 in 20 minutes: a multicenter study. J Clin Microbiol 2021; 59(2):e02811-20. doi: 10.1128/jcm.02811-20 [Crossref] [ Google Scholar]
- Drancourt M, Michel-Lepage A, Boyer S, Raoult D. The point-of-care laboratory in clinical microbiology. Clin Microbiol Rev 2016; 29(3):429-47. doi: 10.1128/cmr.00090-15 [Crossref] [ Google Scholar]
- Pumford EA, Lu J, Spaczai I, Prasetyo ME, Zheng EM, Zhang H. Developments in integrating nucleic acid isothermal amplification and detection systems for point-of-care diagnostics. Biosens Bioelectron 2020; 170:112674. doi: 10.1016/j.bios.2020.112674 [Crossref] [ Google Scholar]
- Chakraborty S. Democratizing nucleic acid-based molecular diagnostic tests for infectious diseases at resource-limited settings–from point of care to extreme point of care. Sens Diagn 2024; 3(4):536-61. doi: 10.1039/d3sd00304c [Crossref] [ Google Scholar]
- Munawar MA. Critical insight into recombinase polymerase amplification technology. Expert Rev Mol Diagn 2022; 22(7):725-37. doi: 10.1080/14737159.2022.2109964 [Crossref] [ Google Scholar]
- Obande GA, Banga Singh KK. Current and future perspectives on isothermal nucleic acid amplification technologies for diagnosing infections. Infect Drug Resist 2020; 13:455-83. doi: 10.2147/idr.S217571 [Crossref] [ Google Scholar]
- Astatke M, Tiburzi O, Connolly A. A novel RNA detection technique for point-of-care identification of pathogens. J Immunoassay Immunochem 2022; 43(2):1955380. doi: 10.1080/15321819.2021.1955380 [Crossref] [ Google Scholar]
- Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis 2011; 52 Suppl 4:S296-304. doi: 10.1093/cid/cir045 [Crossref] [ Google Scholar]
- Wang X, Liu Y, Liu H, Pan W, Ren J, Zheng X. Recent advances and application of whole genome amplification in molecular diagnosis and medicine. MedComm (2020) 2022; 3(1):e116. doi: 10.1002/mco2.116 [Crossref] [ Google Scholar]
- Hodor P, Pope CE, Whitlock KB, Hoffman LR, Limbrick DL, McDonald PJ. Molecular characterization of microbiota in cerebrospinal fluid from patients with CSF shunt infections using whole genome amplification followed by shotgun sequencing. Front Cell Infect Microbiol 2021; 11:699506. doi: 10.3389/fcimb.2021.699506 [Crossref] [ Google Scholar]
- Leonardo S, Toldrà A, Campàs M. Biosensors based on isothermal DNA amplification for bacterial detection in food safety and environmental monitoring. Sensors (Basel) 2021; 21(2):602. doi: 10.3390/s21020602 [Crossref] [ Google Scholar]
- Olatunji AO, Olaboye JA, Maha CC, Kolawole TO, Abdul S. Revolutionizing infectious disease management in low-resource settings: the impact of rapid diagnostic technologies and portable devices. Int J Appl Res Soc Sci 2024; 6(7):1417-32. doi: 10.51594/ijarss.v6i7.1332 [Crossref] [ Google Scholar]
- Zhu H, Zhang H, Xu Y, Laššáková S, Korabečná M, Neužil P. PCR past, present and future. Biotechniques 2020; 69(4):317-25. doi: 10.2144/btn-2020-0057 [Crossref] [ Google Scholar]
- Hassanzadeh Khanmiri H, Yazdanfar F, Mobed A, Rezamohammadi F, Rahmani M, Haghgouei T. Biosensors; noninvasive method in detection of C-reactive protein (CRP). Biomed Microdevices 2023; 25(3):27. doi: 10.1007/s10544-023-00666-y [Crossref] [ Google Scholar]
- Pohanka M. Diagnoses based on C-reactive protein point-of-care tests. Biosensors (Basel) 2022; 12(5):344. doi: 10.3390/bios12050344 [Crossref] [ Google Scholar]
- AlJarhi UM, Sadek KM, Darwish EM, Elmessiery RM, Salem K, Khalil SA. Evaluation of serum presepsin, procalcitonin, copeptin, and high-sensitivity C-reactive protein for differentiating bacterial infection from disease activity in Egyptian patients with systemic lupus erythematosus. Clin Rheumatol 2021; 40(5):1861-9. doi: 10.1007/s10067-020-05471-z [Crossref] [ Google Scholar]
- Ding S, Ma J, Song X, Dong X, Xie L, Song X. Diagnostic accuracy of procalcitonin, neutrophil-to-lymphocyte ratio, and C-reactive protein in detection of bacterial infections and prediction of outcome in nonneutropenic febrile patients with lung malignancy. J Oncol 2020; 2020:2192378. doi: 10.1155/2020/2192378 [Crossref] [ Google Scholar]
- Rajsic S, Breitkopf R, Bachler M, Treml B. Diagnostic modalities in critical care: point-of-care approach. Diagnostics (Basel) 2021; 11(12):2202. doi: 10.3390/diagnostics11122202 [Crossref] [ Google Scholar]
- Park HD. Current status of clinical application of point-of-care testing. Arch Pathol Lab Med 2021; 145(2):168-75. doi: 10.5858/arpa.2020-0112-RA [Crossref] [ Google Scholar]
- Carlton HC, Savović J, Dawson S, Mitchelmore PJ, Elwenspoek MM. Novel point-of-care biomarker combination tests to differentiate acute bacterial from viral respiratory tract infections to guide antibiotic prescribing: a systematic review. Clin Microbiol Infect 2021; 27(8):1096-108. doi: 10.1016/j.cmi.2021.05.018 [Crossref] [ Google Scholar]
- Antoñanzas F, Juárez-Castelló CA, Rodríguez-Ibeas R. Using point-of-care diagnostic testing for improved antibiotic prescription: an economic model. Health Econ Rev 2021; 11(1):29. doi: 10.1186/s13561-021-00326-y [Crossref] [ Google Scholar]
- Yang SM, Lv S, Zhang W, Cui Y. Microfluidic point-of-care (POC) devices in early diagnosis: a review of opportunities and challenges. Sensors (Basel) 2022; 22(4):1620. doi: 10.3390/s22041620 [Crossref] [ Google Scholar]
- Song Q, Sun X, Dai Z, Gao Y, Gong X, Zhou B. Point-of-care testing detection methods for COVID-19. Lab Chip 2021; 21(9):1634-60. doi: 10.1039/d0lc01156h [Crossref] [ Google Scholar]
- Wang C, Liu M, Wang Z, Li S, Deng Y, He N. Point-of-care diagnostics for infectious diseases: from methods to devices. Nano Today 2021; 37:101092. doi: 10.1016/j.nantod.2021.101092 [Crossref] [ Google Scholar]
- Heidt B, Siqueira WF, Eersels K, Diliën H, van Grinsven B, Fujiwara RT. Point of care diagnostics in resource-limited settings: a review of the present and future of PoC in its most needed environment. Biosensors (Basel) 2020; 10(10):133. doi: 10.3390/bios10100133 [Crossref] [ Google Scholar]
- Zamani M, Furst AL, Klapperich CM. Strategies for engineering affordable technologies for point-of-care diagnostics of infectious diseases. Acc Chem Res 2021; 54(20):3772-9. doi: 10.1021/acs.accounts.1c00434 [Crossref] [ Google Scholar]
- Baldi P, La Porta N. Molecular approaches for low-cost point-of-care pathogen detection in agriculture and forestry. Front Plant Sci 2020; 11:570862. doi: 10.3389/fpls.2020.570862 [Crossref] [ Google Scholar]
- Chokkalla AK, Recio BD, Devaraj S. Best practices for effective management of point of care testing. EJIFCC 2023; 34(3):245-9. [ Google Scholar]
- Almuntasheri MH, Altamimi HR, Almayad KA, Alshagageeg BA, Hamdan HM, Alamri MS. Challenges and strategies in point-of-care testing in remote and resource-limited settings. J Surv Fish Sci 2023; 10(5):225-31. doi: 10.53555/jsf.v10i5.2299 [Crossref] [ Google Scholar]
- Nichols JH, Alter D, Chen Y, Isbell TS, Jacobs E, Moore N. AACC guidance document on management of point-of-care testing. J Appl Lab Med 2020; 5(4):762-87. doi: 10.1093/jalm/jfaa059 [Crossref] [ Google Scholar]
- Lisby JG, Schneider UV. Point of care testing for infectious disease: ownership and quality. J Antimicrob Chemother 2021; 76(Suppl 3):iii28-32. doi: 10.1093/jac/dkab247 [Crossref] [ Google Scholar]
- Roy S, Arshad F, Eissa S, Safavieh M, Alattas SG, Ahmed MU. Recent developments towards portable point-of-care diagnostic devices for pathogen detection. Sens Diagn 2022; 1(1):87-105. doi: 10.1039/d1sd00017a [Crossref] [ Google Scholar]
- Khan AI, Pratumvinit B, Jacobs E, Kost GJ, Kary H, Balla J. Point-of-care testing performed by healthcare professionals outside the hospital setting: consensus based recommendations from the IFCC Committee on Point-of-Care Testing (IFCC C-POCT). Clin Chem Lab Med 2023; 61(9):1572-9. doi: 10.1515/cclm-2023-0502 [Crossref] [ Google Scholar]
- Nelson PP, Rath BA, Fragkou PC, Antalis E, Tsiodras S, Skevaki C. Current and future point-of-care tests for emerging and new respiratory viruses and future perspectives. Front Cell Infect Microbiol 2020; 10:181. doi: 10.3389/fcimb.2020.00181 [Crossref] [ Google Scholar]
- Zare Harofte S, Soltani M, Siavashy S, Raahemifar K. Recent advances of utilizing artificial intelligence in lab on a chip for diagnosis and treatment. Small 2022; 18(42):e2203169. doi: 10.1002/smll.202203169 [Crossref] [ Google Scholar]
- Bhaiyya M, Panigrahi D, Rewatkar P, Haick H. Role of machine learning assisted biosensors in point-of-care-testing for clinical decisions. ACS Sens 2024; 9(9):4495-519. doi: 10.1021/acssensors.4c01582 [Crossref] [ Google Scholar]
- Sana F, Isselbacher EM, Singh JP, Heist EK, Pathik B, Armoundas AA. Wearable devices for ambulatory cardiac monitoring: JACC state-of-the-art review. J Am Coll Cardiol 2020; 75(13):1582-92. doi: 10.1016/j.jacc.2020.01.046 [Crossref] [ Google Scholar]
- Zovko K, Šerić L, Perković T, Belani H, Šolić P. IoT and health monitoring wearable devices as enabling technologies for sustainable enhancement of life quality in smart environments. J Clean Prod 2023; 413:137506. doi: 10.1016/j.jclepro.2023.137506 [Crossref] [ Google Scholar]
- Rasti R. Point-of-Care Diagnostics of Childhood Central Nervous System Infections, with a Focus on Usability in Low-Resource Settings [dissertation]. Sweden: Karolinska Institutet; 2022.
- Vaezipour N, Fritschi N, Brasier N, Bélard S, Domínguez J, Tebruegge M. Towards accurate point-of-care tests for tuberculosis in children. Pathogens 2022; 11(3):327. doi: 10.3390/pathogens11030327 [Crossref] [ Google Scholar]