Arch Iran Med. 25(2):127-132.
doi: 10.34172/aim.2022.22
Systematic Review
Regulatory T Cells in Immunopathogenesis and Severity of COVID-19: A Systematic Review
Sam Alahyari 1, 2  , Mohsen Rajaeinejad 3, *
, Mohsen Rajaeinejad 3, *  , Hasan Jalaeikhoo 3, Davar Amani 4
, Hasan Jalaeikhoo 3, Davar Amani 4 
Author information:
1Science and Research Branch, Faculty of Medicine, AJA University of Medical Sciences, Tehran, Iran
2Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3AJA Cancer Epidemiology Research and Treatment Center (AJA‐ CERTC), AJA University of Medical Sciences, Tehran, Iran
4Department of Immunology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
*Corresponding Author: Mohsen Rajaeinejad, MD, Aja University of Medical Sciences, Etemadzadeh St. West Fatemi St. Tehran, Iran. Tel: 982186096350-3, Fax: 982122719014, E-mail:
mrajaei@gmail.com
Abstract
Background:
Severe cases of coronavirus disease 2019 (COVID-19) often experience hyper-inflammatory reactions, acute respiratory distress syndrome (ARDS), blood clotting, and organ damage. The most prominent immunopathology of advanced COVID-19 is cytokine release syndrome, or "cytokine storm" which is attributed to a defect of immune-regulating mechanisms. This study aimed to evaluate the role of regulatory T cells (Tregs) as one of the main cells that maintain immune homeostasis.
Methods:
A systematic search was performed on PubMed, Scopus and Google Scholar. All English articles related to Treg’s role in COVID-19 were extracted and evaluated by two researchers independently. Study eligibility was assessed based on modified Evidence-based librarianship (EBL) checklist.
Results:
Nineteen eligible studies comparing Treg cells in COVID-19 patients with the control group or comparing alterations of this cell in severe and moderate patients were evaluated. Currently, there is no consensus regarding the increase or decrease of Tregs in COVID-19 patients compared to the control group. However, it was observed that Tregs in severe COVID-19 patients were significantly lower than moderate patients, resulting in uncontrolled inflammation and cytokine storm.
Conclusion:
Regulatory T cells can be one of the determinants of disease severity and prognosis in patients with COVID-19 by inhibiting rampant inflammation and preventing cytokine storms.
Keywords: COVID-19, Immune response, Regulatory T cell, SARS-CoV-2, Severity
Copyright and License Information
© 2022 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Alahyari S, Rajaeinejad M, Jalaeikhoo H, Aman D. Regulatory t cells in immunopathogenesis and severity of covid-19: a systematic review. Arch Iran Med. 2022;25(2):127-132. doi: 10.34172/aim.2022.22
Introduction
T cells need to induce SARS-CoV-2-specific immune responses by detecting viral antigens through antigen receptors (TCR). By recognizing antigens as peptides attached to the major histocompatibility complex, T cells can recognize not only structural proteins such as spike (S) and nucleocapsid (N) but also nonstructural proteins including ORF3a and ORF7. Once a viral antigen is detected, CD4 + T cells are activated and can be differentiated into helper T cell subsets through the activity of transcription factors and specific cytokines for each subset.1 However, in some diseases, such as coronavirus disease 2019 (COVID-19), the development of uncontrolled inflammation by the immune system with overproduction of pro-inflammatory cytokines including interleukin‐1β (IL‐1β), IL‐6, tumor necrosis factor‐α (TNF‐ α), and IL‐8 can lead to mortality.2,3 Tregs are commonly defined by the forkhead box protein 3 (FoxP3) as CD3+ CD4+ CD25highCD127low FoxP3+ T cells and are classically known for development of immune tolerance and preventing autoimmune diseases.4 Numerous publications have observed that impaired production and performance of Tregs are associated with greater disease severity, as reported in patients with various inflammatory diseases. Tregs can limit effector T-cell function. The most critical population of Tregs, which expresses Foxp3, can limit the activation, proliferation, and effector roles in series of immune cells.5-7 Studies have shown that the coronavirus and arterivirus lead to an increase in CD4 + CD25 + FoxP3 + lymphocytes. However, Treg activation results are different for each virus.8 Since data were limited for Treg alterations for different severities of COVID-19 and its comparison between patients and controls, the aim of this study was to evaluate the alterations of Treg for different severities of COVID-19 and also the differences of this cell in patients and the control group.
Materials and Methods
We searched PubMed, Google Scholar and Scopus databases until May 1, 2021. Regulatory T-cells, Treg cells, Regulatory T-Lymphocytes, Regulatory T Lymphocytes, Th3 cells, and CD4 + CD25 + cells keywords were used to search databases. Also, the keywords for COVID-19 included: COVID-19, 2019-nCoV, 2019-nCoV infection, 2019-nCoV disease, 2019 novel corona virus, 2019 novel coronavirus infection, 2019 novel coronavirus disease, coronavirus disease 2019, coronavirus disease 2019 virus, 2019 novel coronavirus infection or coronavirus disease-19. All English-language studies evaluating Treg changes in COVID-19 patients were reviewed and no data limitations were applied. The search was conducted by two researchers independently and the consistency of the results was high. In contradictory cases, the third person reviewed the articles. For greater coherence of the results, mild and extremely severe patients were categorized into moderate and severe disease group, respectively (Supplementary file 1, Table S1).
Inclusion and Exclusion Criteria
All English-language studies were included with the following criteria: cross-sectional, case-control, and cohort studies that evaluated at least two of the following markers: CD4, FoxP3, CD25, and CD127. Studies that identified COVID-19 by real-time PCR were evaluated. All preprint articles, books, congresses, and duplicate studies were removed. In addition, case reports were removed due to the small sample size and the low accuracy of the results.
Eligibility Assessment
Based on the Evidence-based librarianship (EBL) criteria,9 we created a checklist to evaluate the studies. In this questionnaire, there were three answers to the questions: “yes”, “no” and “unclear”. The total number of questions was 18. If the “yes” answer to the total questions for an article was less than 75%, it would be excluded from the study due to its low validity.
Results
Initially, 1352 studies were obtained. After deleting the duplicates, 319 articles remained. After initial evaluation, theses, books, and abstracts for conferences were removed. A total of 211 articles that met the study criteria were evaluated more precisely. Among them, 176 were review articles (often with subjects of Treg or COVID-19), 6 studies did not have a definition of Treg, 4 were commentary studies and 6 were case-reports. Finally, 19 articles that fully met the inclusion criteria of the study were reviewed (Figure 1).
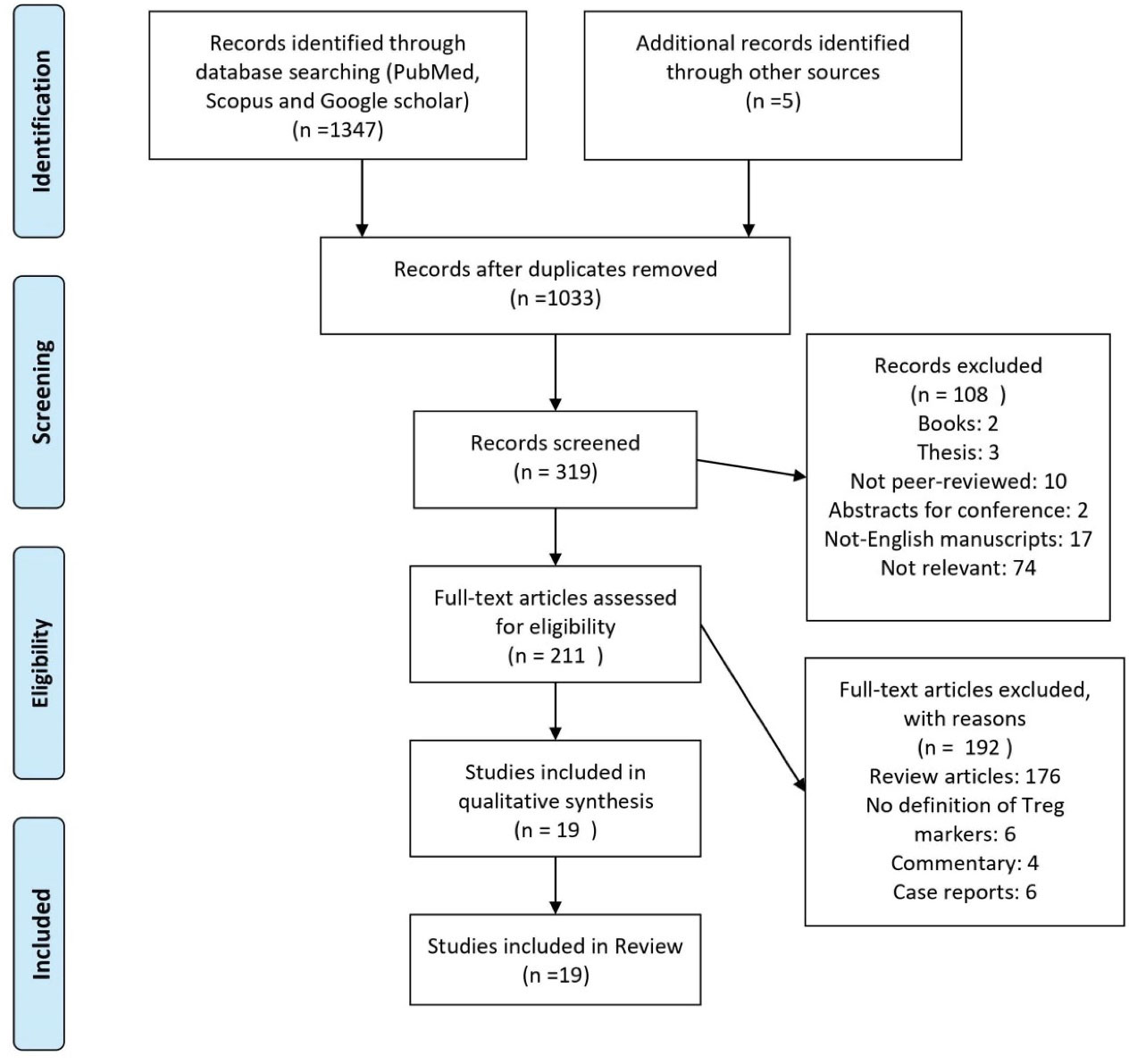
Figure 1.
Flowchart of the Evaluated Studies.
.
Flowchart of the Evaluated Studies.
Comparison of Treg Cells in Patients vs. Controls
There was a controversy in the comparison of Treg cells in patients with COVID-19 and the control group (Table 1). Some studies found that these cells increased significantly in COVID-19 patients compared to the control group.10,11 In one study, it was also observed that the number of regulatory cells in moderate patients, in contrast to severe patients, increased significantly compared to controls.12 Opposite results were reported in other studies, which indicated a sharp increase in these cells in severe patients.13,14 Finally, Song et al observed that the increase in regulatory cells in severe and moderate patients was not significant compared to the control group.15
Table 1.
Studies That Compared Tregs in COVID-19 Patients with Control Group
|
First Author
|
Number of Patients
|
Regulatory T Cell Markers
|
P Treg Patients/Control
|
P Treg Moderate/Control
|
P Treg Severe/Control
|
| Chen 10 |
102 |
CD4+CD25+CD127low |
<0.05 |
<0.001 |
<0.001 |
| De Biasi 11 |
39 |
CD4+CD25+CD127low |
<0.05 |
NM |
NM |
| Tan 12 |
56 |
CD3+CD4+CD25+CD127low |
NM |
<0.01 |
>0.05 |
| Vigón 13 |
109 |
CD4+CD25+CD127low |
>0.05 |
>0.05 |
<0.01 |
| Rendeiro 14 |
36 |
CD4+CD25+CD127low |
>0.05 |
>0.05 |
<0.01 |
| Song 15 |
41 |
CD4+CD25+CD127low |
>0.05 |
>0.05 |
>0.05 |
| Mohebbi 16 |
30 |
CD4+FoxP3+ |
<0.001 |
NM |
NM |
| Mahmoud Salehi Khesht 17 |
60 |
CD4+FoxP3+ |
NM |
<0.05 |
<0.01 |
| Schub 18 |
50 |
CD4+CD25+CD127low |
NM |
>0.05 |
<0.01 |
| Laing 19 |
63 |
CD4+CD25+CD127low |
NM |
0.013 |
0.001 |
| Sadeghi 20 |
40 |
CD4+FoxP3+ |
<0.001 |
NM |
<0.001 |
| Jia 21 |
19 |
CD4+CD25+CD127low |
0.027 |
NM |
NM |
| Kang 22 |
12 |
CD4+FoxP3+ |
NM |
0.24 |
0.65 |
In contrast, in some studies, a decrease of Tregs in patients compared to controls was reported. Mohebbi et al found that the number of these cells was significantly reduced in patients with COVID-19.16 Mahmoud Salehi Khesht et al also observed that the number of regulatory T cells in both moderate and severe patients decreased significantly compared to the control.17 These results were in line with the results of other studies.18-20 The results of an evaluation in children with COVID-19 also showed that Treg cells decreased sharply in the acute phase of the disease and increased again in the convalescent phase (11–27 days after the onset of the disease).21 An insignificant decrease in Tregs in patients compared to controls was also reported in one study.22
Comparison of Treg in Severe Patients vs. Moderate Patients
Although there was discordance in the results of assessment of this case among the studies, the most significant change was the decrease in Tregs in severe patients compared to the moderate ones (Table 2). Qin et al reported a significant reduction in Tregs in severe COVID-19 patients compared to moderate patients.23 Similar results were observed in some other studies.17,18,24 In contrast, Del Bello et al found a significant increase in Treg cell counts in severe compared to moderate COVID-19 patients who had undergone solid organ transplantation (SOT).25
Table 2.
Studies That Compared Tregs in Severe and Moderate COVID-19 Patients
|
First Author
|
Number of Patients
|
Number of Moderate Patients
|
Number of Severe Patients
|
Regulatory T Cell Markers
|
P Treg Severe/Moderate
|
| Chen 10 |
21 |
10 |
11 |
CD4+CD25+CD127low |
>0.05 |
| Tan 12 |
56 |
31 |
25 |
CD3+CD4+CD25+CD127low |
>0.05 |
| Song 15 |
41 |
29 |
12 |
CD4+CD25+CD127low |
>0.05 |
| Mahmoud Salehi Khesht 17 |
60 |
30 |
30 |
CD4+FoxP3+ |
<0.05 |
| Schub 18 |
50 |
36 |
14 |
CD4+CD25+CD127low |
<0.001 |
| Laing 19 |
63 |
32 |
31 |
CD4+CD25+CD127low |
>0.05 |
| Kang 22 |
12 |
8 |
4 |
CD4+FoxP3+ |
0.83 |
| Qin 23 |
44 |
17 |
27 |
CD3+CD4+CD25+CD127low |
0.04 |
| Meckiff 24 |
40 |
18 |
22 |
CD4+FoxP3+ |
<0.001 |
| Del Bello Bello25 |
51 |
29 |
22 |
CD4+CD25+CD127low |
0.02 |
| Jiang 26 |
103 |
86 |
17 |
CD4+CD25+CD127low |
0.85 |
| Chen 27 |
21 |
10 |
11 |
CD4+CD25+CD127low |
0.9 |
| Wang 28 |
65 |
30 |
35 |
CD4+CD25+CD127low |
>0.05 |
Treg alterations reported in other evaluated studies were insignificant.10,12,15,19,22,26-28 However, in two studies, the CD45RA + Treg cell population was assessed in moderate and severe COVID-19 patients. In both studies, it was observed that the number of these cells in the more severe disease was significantly reduced compared to milder disease.27,28
Discussion
In this review, we evaluated the trend of Treg changes in COVID-19 patients compared to the control group at different clinical stages of the disease. Controversy was observed in the comparison of Tregs in COVID-19 patients compared to the control group which can be attributed to various reasons. This should be mainly due to the use of different markers in identifying Tregs because CD25 +/hi, CD127-/lo, FoxP3 + and their various combinations were used. In addition, the number of Tregs likely varies in patients with COVID-19 at different stages or with different disease intensities. CD25+/hi and CD127-/lo are just surrogate surface markers of FoxP3-expressing Tregs. Therefore, CD4 and FoxP3 markers are recommended to identify Tregs in future studies.29,30 FOXP3 is induced in activated T cells by TCR signals and its transcription is further enhanced by IL-2 and TGF-b signaling. After expression in T cells, FOXP3 proteins bind to pre-existing transcription factors, particularly RUNX1 and ETS1, thereby transforming systems containing RUNX1-ETS1 for T cell activation and effective immune regulatory function.31,32
Regarding the comparison of Treg cell counts in severe and moderate COVID-19 patients, it was observed in most studies that these cells had a significant decrease in severe patients compared to moderate ones. It has been previously reported that in viral respiratory infections, Treg cells can prevent the cytokine storm, reducing the severity of viral pneumonia and acute lung injury.33,34 In acute human and rat lung injury, Tregs accumulation is associated with reduction of immunopathology by inhibiting innate immune responses.35 Therefore, Treg cells in patients with COVID-19 are more likely to prevent hyper-inflammation and cytokine storms. Significant reduction of Treg cells in these patients could be due to direct destruction of these cells by the virus, induction of apoptosis in activated cells, sequestration in infected tissue, and the inhibitory effect of IL-6 on Treg cells.36-39 This is probably one of the reasons why IL-6 levels can be used to predict disease severity.40 Decreased Treg cells upset the balance between regulatory and effector cells, leading to an uncontrolled inflammatory response, followed by local and systemic tissue damage.41-44 Decreased proportion and dysfunction of Treg are of paramount importance in COVID-19-induced injury.45-46 Treg cells can prevent cytokine storms, accelerate the healing process of acute respiratory distress syndrome (ARDS), and suppress the development of inflammatory disorders of the lung.47-49 Treg cells prevent ARDS and cytokine storms through different mechanisms. These mechanisms include production of immunosuppressive cytokines (IL-10, IL-35 and TGFβ), IL-2 consumption, induction of death in effective cells through granzyme and perforin, inhibition of activation of antigen-presenting cells (APC) and metabolic disruption (such as adenosine production).50 Thus, although Chen et al10 reported a significant increase in Treg in patients compared to controls, a significant decrease in IL-10 in severe patients compared to controls may be due to the dysfunction of these cells despite an increase in their number. Also, in an experimental model of acute lung injury in mice, it was shown that infiltration of Treg cells in bronchoalveolar lavage fluid helps to resolve lung damage by inducing neutrophil apoptosis, macrophage efferocytosis, and decreased fibrocyte recruitment.51 A study by Gladstone et al on the successful treatment of patients with COVID-19 and ARDS using allogeneic Tregs confirms the importance of Treg deficiency in the pathogenesis of COVID-19 and the potential therapeutic benefits of Treg.52
Bello et al observed that the number of Treg cells in SOT patients with severe COVID-19 increased significantly compared to moderate patients. Some studies have shown that Treg increases in SOT patients in order to increase the immune tolerance of the transplanted organ.53-55 However, the increase in these cells in SOT patients with severe COVID-19 requires close investigation. Based on these various observations, it can be assumed that the delay and weakness of specific T cells and the neutralization of the humoral response to SARS-CoV-2 due to immune suppression lead to virus escape from immune neutralization and prevent rapid clearance of the virus, resulting in severe disease.56
In conclusion, the reported Treg cell alterations and function in COVID-19 are controversial. Although comparing these alterations between COVID-19 patients and healthy individuals in the studies yielded different results, it seems that in most studies, in more severe disease, a significant decrease in Tregs has been reported which disrupts immune homeostasis and creates a cytokine storm. However, early activation of this cell may also suppress the immune response and cause severe disease. However, the success of allogeneic Treg therapy could confirm the reduction of this cell in severe disease and the occurrence of rampant inflammatory response. Therefore, in order to more accurately evaluate the role of Treg cells in COVID-19, large studies are needed to compare the changes of this cell in COVID-19 patients and healthy individuals, as well as severe and moderate patients. Also, comparing the level of cytokines produced by Treg (such as IL-10) at different clinical stages of COVID-19 as well as with the control group, can evaluate the function of this cell (regardless of its decreasing or increasing number).
Supplementary Materials
Supplementary file 1 contains Table S1.
(pdf)
Acknowledgements
We would like to acknowledge Dr. Leila Chegini for her comments on this manuscript.
Authors’ Contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SA, MR and HJ. The first draft of the manuscript was written by SA, MR and DA. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript
Conflict of Interest Disclosures
The authors declare that they have no conflict of interest.
Ethical Statement
The study was performed in accordance with Declaration of Helsinki and was approved by Shahid Beheshti University of Medical Sciences ethical committee (ethical code: IR.AJAUMS.REC.1399.062)
References
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181(7):1489-501. doi: 10.1016/j.cell.2020.05.015 [Crossref] [ Google Scholar]
- McKechnie JL, Blish CA. The innate immune system: fighting on the front lines or fanning the flames of COVID-19?. Cell Host Microbe 2020; 27(6):863-9. doi: 10.1016/j.chom.2020.05.009 [Crossref] [ Google Scholar]
- Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med 2020; 217(6):e20200678. doi: 10.1084/jem.20200678 [Crossref] [ Google Scholar]
- Plitas G, Rudensky AY. Regulatory T cells: differentiation and function. Cancer Immunol Res 2016; 4(9):721-5. doi: 10.1158/2326-6066.cir-16-0193 [Crossref] [ Google Scholar]
- Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol 2015; 16(2):188-96. doi: 10.1038/ni.3077 [Crossref] [ Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001; 27(1):20-1. doi: 10.1038/83713 [Crossref] [ Google Scholar]
- Sakaguchi S, Wing K, Yamaguchi T. Dynamics of peripheral tolerance and immune regulation mediated by Treg. Eur J Immunol 2009; 39(9):2331-6. doi: 10.1002/eji.200939688 [Crossref] [ Google Scholar]
- Cecere TE, Todd SM, Leroith T. Regulatory T cells in arterivirus and coronavirus infections: do they protect against disease or enhance it?. Viruses 2012; 4(5):833-46. doi: 10.3390/v4050833 [Crossref] [ Google Scholar]
- Glynn L. A critical appraisal tool for library and information research. Libr Hi Tech 2006; 24(3):387-99. doi: 10.1108/07378830610692154 [Crossref] [ Google Scholar]
- Chen X, Huang J, Huang Y, Chen J, Huang Y, Jiang X. Characteristics of immune cells and cytokines in patients with coronavirus disease 2019 in Guangzhou, China. Hum Immunol 2020; 81(12):702-8. doi: 10.1016/j.humimm.2020.08.006 [Crossref] [ Google Scholar]
- De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun 2020; 11(1):3434. doi: 10.1038/s41467-020-17292-4 [Crossref] [ Google Scholar]
- Tan M, Liu Y, Zhou R, Deng X, Li F, Liang K. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 2020; 160(3):261-8. doi: 10.1111/imm.13223 [Crossref] [ Google Scholar]
- Vigón L, Fuertes D, García-Pérez J, Torres M, Rodríguez-Mora S, Mateos E. Impaired cytotoxic response in PBMCs from patients with COVID-19 admitted to the ICU: biomarkers to predict disease severity. Front Immunol 2021; 12:665329. doi: 10.3389/fimmu.2021.665329 [Crossref] [ Google Scholar]
- Rendeiro AF, Casano J, Vorkas CK, Singh H, Morales A, DeSimone RA. Profiling of immune dysfunction in COVID-19 patients allows early prediction of disease progression. Life Sci Alliance 2021; 4(2):e202000955. doi: 10.26508/lsa.202000955 [Crossref] [ Google Scholar]
- Song JW, Zhang C, Fan X, Meng FP, Xu Z, Xia P. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun 2020; 11(1):3410. doi: 10.1038/s41467-020-17240-2 [Crossref] [ Google Scholar]
- Mohebbi SR, Baghaei K, Rostami-Nejad M, Nazemalhosseini Mojarad E, Mirjalali H, Yadegar A. Significant changes of CD4, FOXP3, CD25, and IL6 expression level in Iranian COVID-19 patients. Gastroenterol Hepatol Bed Bench 2020; 13(4):388-92. [ Google Scholar]
- Mahmoud Salehi Khesht A, Karpisheh V, Qubais Saeed B, Olegovna Zekiy A, Yapanto LM, Nabi Afjadi M. Different T cell related immunological profiles in COVID-19 patients compared to healthy controls. Int Immunopharmacol 2021; 97:107828. doi: 10.1016/j.intimp.2021.107828 [Crossref] [ Google Scholar]
- Schub D, Klemis V, Schneitler S, Mihm J, Lepper PM, Wilkens H. High levels of SARS-CoV-2-specific T cells with restricted functionality in severe courses of COVID-19. JCI Insight 2020; 5(20):e142167. doi: 10.1172/jci.insight.142167 [Crossref] [ Google Scholar]
- Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 2020; 26(10):1623-35. doi: 10.1038/s41591-020-1038-6 [Crossref] [ Google Scholar]
- Sadeghi A, Tahmasebi S, Mahmood A, Kuznetsova M, Valizadeh H, Taghizadieh A. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J Cell Physiol 2021; 236(4):2829-39. doi: 10.1002/jcp.30047 [Crossref] [ Google Scholar]
- Jia R, Wang X, Liu P, Liang X, Ge Y, Tian H. Mild cytokine elevation, moderate CD4+ T cell response and abundant antibody production in children with COVID-19. Virol Sin 2020; 35(6):734-43. doi: 10.1007/s12250-020-00265-8 [Crossref] [ Google Scholar]
- Kang CK, Han GC, Kim M, Kim G, Shin HM, Song KH. Aberrant hyperactivation of cytotoxic T-cell as a potential determinant of COVID-19 severity. Int J Infect Dis 2020; 97:313-21. doi: 10.1016/j.ijid.2020.05.106 [Crossref] [ Google Scholar]
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020; 71(15):762-8. doi: 10.1093/cid/ciaa248 [Crossref] [ Google Scholar]
- Meckiff BJ, Ramírez-Suástegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+ T cells in COVID-19. Cell 2020; 183(5):1340-53. doi: 10.1016/j.cell.2020.10.001 [Crossref] [ Google Scholar]
- Del Bello A, Kamar N, Vergez F, Faguer S, Marion O, Beq A. Adaptive lymphocyte profile analysis discriminates mild and severe forms of COVID-19 after solid organ transplantation. Kidney Int 2021; 100(4):915-27. doi: 10.1016/j.kint.2021.05.032 [Crossref] [ Google Scholar]
- Jiang M, Guo Y, Luo Q, Huang Z, Zhao R, Liu S. T-cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of coronavirus disease 2019. J Infect Dis 2020; 222(2):198-202. doi: 10.1093/infdis/jiaa252 [Crossref] [ Google Scholar]
- Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130(5):2620-9. doi: 10.1172/jci137244 [Crossref] [ Google Scholar]
- Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 2020; 5(10):e137799. doi: 10.1172/jci.insight.137799 [Crossref] [ Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4(4):330-6. doi: 10.1038/ni904 [Crossref] [ Google Scholar]
- Simonetta F, Chiali A, Cordier C, Urrutia A, Girault I, Bloquet S. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol 2010; 40(9):2528-38. doi: 10.1002/eji.201040531 [Crossref] [ Google Scholar]
- Ono M. Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes. Immunology 2020; 160(1):24-37. doi: 10.1111/imm.13178 [Crossref] [ Google Scholar]
- Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature 2007; 446(7136):685-9. doi: 10.1038/nature05673 [Crossref] [ Google Scholar]
- Veiga-Parga T, Sehrawat S, Rouse BT. Role of regulatory T cells during virus infection. Immunol Rev 2013; 255(1):182-96. doi: 10.1111/imr.12085 [Crossref] [ Google Scholar]
- Lin S, Wu H, Wang C, Xiao Z, Xu F. Regulatory T cells and acute lung injury: cytokines, uncontrolled inflammation, and therapeutic implications. Front Immunol 2018; 9:1545. doi: 10.3389/fimmu.2018.01545 [Crossref] [ Google Scholar]
- D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 2009; 119(10):2898-913. doi: 10.1172/jci36498 [Crossref] [ Google Scholar]
- Li T, Qiu Z, Zhang L, Han Y, He W, Liu Z. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis 2004; 189(4):648-51. doi: 10.1086/381535 [Crossref] [ Google Scholar]
- Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020; 9(1):727-32. doi: 10.1080/22221751.2020.1746199 [Crossref] [ Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441(7090):235-8. doi: 10.1038/nature04753 [Crossref] [ Google Scholar]
- Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010; 40(7):1830-5. doi: 10.1002/eji.201040391 [Crossref] [ Google Scholar]
- Vyas AK, Varma V, Garg G, Gupta P, Trehanpati N. The role and delicate balance of Host Immunity in Coronavirus Disease-19. Iran J Immunol 2021; 18(1):1-12. doi: 10.22034/iji.2021.88526.1874 [Crossref] [ Google Scholar]
- Muyayalo KP, Huang DH, Zhao SJ, Xie T, Mor G, Liao AH. COVID-19 and Treg/Th17 imbalance: potential relationship to pregnancy outcomes. Am J Reprod Immunol 2020; 84(5):e13304. doi: 10.1111/aji.13304 [Crossref] [ Google Scholar]
- Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 2014; 13(6):668-77. doi: 10.1016/j.autrev.2013.12.004 [Crossref] [ Google Scholar]
- Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules 2017; 22(1):134. doi: 10.3390/molecules22010134 [Crossref] [ Google Scholar]
- Álvarez-Rodríguez L, Martínez-Taboada V, Calvo-Alén J, Beares I, Villa I, López-Hoyos M. Altered Th17/Treg ratio in peripheral blood of systemic lupus erythematosus but not primary antiphospholipid syndrome. Front Immunol 2019; 10:391. doi: 10.3389/fimmu.2019.00391 [Crossref] [ Google Scholar]
- Trandem K, Anghelina D, Zhao J, Perlman S. Regulatory T cells inhibit T cell proliferation and decrease demyelination in mice chronically infected with a coronavirus. J Immunol 2010; 184(8):4391-400. doi: 10.4049/jimmunol.0903918 [Crossref] [ Google Scholar]
- de Aquino MT, Puntambekar SS, Savarin C, Bergmann CC, Phares TW, Hinton DR. Role of CD25+ CD4+ T cells in acute and persistent coronavirus infection of the central nervous system. Virology 2013; 447(1-2):112-20. doi: 10.1016/j.virol.2013.08.030 [Crossref] [ Google Scholar]
- Gogishvili T, Langenhorst D, Lühder F, Elias F, Elflein K, Dennehy KM. Rapid regulatory T-cell response prevents cytokine storm in CD28 superagonist treated mice. PLoS One 2009; 4(2):e4643. doi: 10.1371/journal.pone.0004643 [Crossref] [ Google Scholar]
- McKinley L, Logar AJ, McAllister F, Zheng M, Steele C, Kolls JK. Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia. J Immunol 2006; 177(9):6215-26. doi: 10.4049/jimmunol.177.9.6215 [Crossref] [ Google Scholar]
- Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S. A distinct function of regulatory T cells in tissue protection. Cell 2015; 162(5):1078-89. doi: 10.1016/j.cell.2015.08.021 [Crossref] [ Google Scholar]
- Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol 2012; 3:51. doi: 10.3389/fimmu.2012.00051 [Crossref] [ Google Scholar]
- Wang L, Zhao L, Lv J, Yin Q, Liang X, Chu Y. BLT1-dependent alveolar recruitment of CD4+CD25+ Foxp3+ regulatory T cells is important for resolution of acute lung injury. Am J Respir Crit Care Med 2012; 186(10):989-98. doi: 10.1164/rccm.201202-0261OC [Crossref] [ Google Scholar]
- Gladstone DE, Kim BS, Mooney K, Karaba AH, D’Alessio FR. Regulatory T cells for treating patients with COVID-19 and acute respiratory distress syndrome: two case reports. Ann Intern Med 2020; 173(10):852-3. doi: 10.7326/l20-0681 [Crossref] [ Google Scholar]
- Mederacke YS, Vondran FW, Kollrich S, Schulde E, Schmitt R, Manns MP. Transient increase of activated regulatory T cells early after kidney transplantation. Sci Rep 2019; 9(1):1021. doi: 10.1038/s41598-018-37218-x [Crossref] [ Google Scholar]
- Issa F, Wood KJ. CD4+ regulatory T cells in solid organ transplantation. Curr Opin Organ Transplant 2010; 15(6):757-64. doi: 10.1097/MOT.0b013e32834017ae [Crossref] [ Google Scholar]
- Atif M, Conti F, Gorochov G, Oo YH, Miyara M. Regulatory T cells in solid organ transplantation. Clin Transl Immunology 2020; 9(2):e01099. doi: 10.1002/cti2.1099 [Crossref] [ Google Scholar]
- Clark SA, Clark LE, Pan J, Coscia A, McKay LGA, Shankar S. SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms. Cell 2021; 184(10):2605-17. doi: 10.1016/j.cell.2021.03.027 [Crossref] [ Google Scholar]