Arch Iran Med. 25(8):564-573.
doi: 10.34172/aim.2022.90
Systematic Review
Objectives, Outcomes, Facilitators, and Barriers of Telemedicine Systems for Patients with Alzheimer’s Disease and their Caregivers and Care Providers: A Systematic Review
Parasto Amiri 1  , Zahra Niazkhani 2, Habibollah Pirnejad 3, 4, Mahdie ShojaeiBaghini 5, Kambiz Bahaadinbeigy 5, *
, Zahra Niazkhani 2, Habibollah Pirnejad 3, 4, Mahdie ShojaeiBaghini 5, Kambiz Bahaadinbeigy 5, * 
Author information:
1Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran
2Nephrology and Kidney Transplant Research Center, Clinical Research Institute, Urmia University of Medical Sciences, Urmia, Iran
3Patient Safety Research Center, Clinical Research Institute, Urmia University of Medical Sciences, Urmia, Iran
4Erasmus School of Health Policy and Management, Erasmus University Rotterdam, Rotterdam, The Netherlands
5Medical Informatics Research Center, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran
Abstract
Background:
Alzheimer’s disease is an extremely expensive chronic disease, which is rapidly becoming a major cause of mortality in adults. For over two decades, telemedicine has been used to assist patients and their caregivers to manage this disease. The present study aimed to evaluate the objectives, outcomes, facilitators, and barriers influencing the use of telemedicine systems for patients with Alzheimer’s disease and their caregivers and care providers.
Methods:
In this systematic review, we searched for the original articles published in databases such as PubMed, Web of Science, and Scopus until November 2021 using relevant keywords. A qualitative content analysis was performed the based on the theory of planned behavior and the health belief model using the ATLAS.ti software.
Results:
In total, 1191 articles were identified, and 60 articles were included in this study. While having different objectives, most of the studies compared telemedicine systems to in-person visits (21.43%) and assessed the feasibility of the implemented method (16.07%). The overall outcomes of telemedicine in the articles were classified as cost-effectiveness (e.g., reduced commute, fuel, and time to access care), clinical outcomes (e.g., lower anxiety, stress, and depression), and patient, caregiver, and healthcare provider satisfaction. In total, 19 facilitators and 12 barriers influencing the use of telemedicine for patients with Alzheimer’s disease and their caregivers were identified.
Conclusion:
According to the results, telemedicine systems could be implemented for various reasons. Developing a clear framework of the drivers and barriers before the implementation of these systems could improve decision-making prior to the design and implementation of telemedicine systems.
Keywords: Alzheimer’s disease, Barriers, Caregivers, Drivers, Telemedicine, Systematic review
Copyright and License Information
© 2022 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Amiri P, Niazkhani Z, Pirnejad H, ShojaeiBaghini M, Bahaadinbeigy K. Objectives, outcomes, facilitators, and barriers of telemedicine systems for patients with alzheimer’s disease and their caregivers and care providers: a systematic review. Arch Iran Med. 2022;25(8):564-573. doi: 10.34172/aim.2022.90
Introduction
In the 21st century, aging is associated with multiple age-specific diseases, such as dementia.1 Studies show that about 11% of the individuals aged more than 65 years are diagnosed with dementia throughout the world, and more than one-third of the people aged more than 85 years tend to experience this condition.2 In a report by the World Health Organization (WHO) in 2008, dementia was recognized as a priority condition in the WHO mental health plan.3 Based on the prediction of the WHO in 2015, the number of the patients with dementia will reach 74 million in 2030.1
Alzheimer’s disease is the most common cause of dementia,4 and the WHO has predicted that Alzheimer’s disease will become the fourth cause of mortality in the world by 2050.5 With disease progression, patients with Alzheimer’s disease become unable to take care of their daily affairs alone.6 Consequently, they require a greater level of care at the advanced stages of the disease, which is time-consuming and costly.7
According to statistics, the costs imposed by Alzheimer’s disease would increase throughout Europe from $177.2 billion to over $250 billion during 2008–2030.7,8 Today, more than 16 million family members provide an estimated 18.6 billion hours of care to patients with Alzheimer’s disease.9 In addition to the high costs and waste of time for care of patients with Alzheimer’s disease, physical and psychological problems have been detected in their caregivers, which are also important health concerns. Therefore, the caregivers of patients with Alzheimer’s disease require constant support to prevent the increased prevalence of depression, anxiety, and occupational burnouts from the moment of diagnosis until the patients’ death.6,10
Given the need for the continuous care of patients with Alzheimer’s disease by the caregivers, barriers such as the unavailability of specialists and medical facilities in remote areas, and the time and costs of transporting the patients to the nearest health center/hospital, supporting these caregivers in-person and by conventional methods is extremely complicated and even impossible.
Today, technology could be used to transfer support and care to the most remote areas. For instance, telemedicine contributes to the support of patients with Alzheimer’s disease and their caregivers. Recently, wide-ranging medical services could be provided in hospitals, outpatient wards, and physician offices through telemedicine. The WHO telemedicine policy in relation to the development of the health-for-all strategy was defined and developed in 1997.11 Accordingly, telemedicine is defined as the delivery of healthcare services where distance is a critical factor by all healthcare professionals using information and communication technologies for the exchange of valid information for the diagnosis, treatment, and prevention of diseases and injuries, as well as research and evaluation and for the continuous education of the healthcare providers in the interest of advancing the health of individuals and their communities.
Recently, telemedicine has been established and considered in numerous areas in developed and developing countries in order to support patients with Alzheimer’s disease and their caregivers.12-14 In a research conducted in the United States (2010), telemedicine was identified as a cost-effective method for patients with Alzheimer’s disease with lower costs than in-person visits.15 Moreover, telemedicine has been shown to increase patient satisfaction through reducing their stress and anxiety, while also decreasing the caregivers’ workload as there would be no need for the displacement of patients for an in-person visit.16 Given the benefits of telemedicine and the issues associated with the care of patients with Alzheimer’s disease and their caregivers, this technology is expected to effectively help these individuals. Despite the positive impacts of telemedicine on patients, some challenges have been attributed to this approach, as well.
In a survey performed in 2012, cultural, managerial, technical, and monitoring problems were among the barriers to telemedicine implementation.17 In some studies, technical obstacles have been reported to significantly affect the establishment or use of telemedicine systems.9,11,13,18-21 Furthermore, professional, legal, and financial obstacles have been highlighted in this regard, with legal and financial barriers known to be of a managerial nature.22 In developed and developing countries, these barriers may prevent rapid expansion of telemedicine in healthcare environments.
In addition to the mentioned issues, developing countries experience communication infrastructure problems (e.g., poor internet speed) in the establishment of these systems.23 Several studies have systematically assessed the barriers to telemedicine in some countries.24-26 To the best of our knowledge, some systematic studies have also focused on telemedicine for patients with Alzheimer’s disease and their caregivers, while none has assessed the drivers and barriers to these systems.27,28 With this background, the present study aimed to systematically review the outcomes and objectives of the studies on the use of telemedicine for patients with Alzheimer’s disease and their caregivers, and the second section of the article is dedicated to the drivers and barriers influencing the development and implementation of this method.
Materials and Methods
This systematic review was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).29
Search Strategy
A literature search was performed in databases such as Scopus, PubMed, and Web of Science for the relevant articles published in English until November 2021. We used various keyword combinations in the titles and abstracts of the articles, including (Alzheimer’s disease) OR (dementia) OR (cognitive impairment)) AND (telemedicine) OR (tele-medicine) OR (telehealth) OR (Tele-health) OR (telecare) OR (telemonitoring) OR (telehomecare) OR (remote monitoring) OR (teleconsult) OR (remote consult)). Table 1 shows the complete search strategy.
Table 1.
Search Strategies in PubMed, Scopus, and Web of Science Databases
|
Database
|
Search Strategies
|
| PubMed |
(((Alzheimer's Disease [MeSH]) OR (dementia [MeSH])) OR (cognitive impairment [Title/Abstract])) AND (((((((((((Telemedicine [MeSH]) OR (Tele-medicine [Title/Abstract])) OR (Telehealth [Title/Abstract])) OR (Tele-health [Title/Abstract])) OR (Telecare [Title/Abstract])) OR (Telemonitoring [Title/Abstract])) OR (Telehomecare [Title/Abstract])) OR (Remote monitoring [Title/Abstract])) OR (teleconsult [Title/Abstract])) OR (remote consult [Title/Abstract]))) |
| Scopus |
( ( TITLE-ABS-KEY ( "Alzheimer's Disease" ) OR TITLE-ABS-KEY ( dementia ) OR TITLE-ABS-KEY ( "cognitive impairment" ) ) ) AND ( ( TITLE-ABS-KEY ( telemedicine ) OR TITLE-ABS-KEY ( "Tele-medicine" ) OR TITLE-ABS-KEY ( telehealth ) OR TITLE-ABS-KEY ( "Tele-health" ) OR TITLE-ABS-KEY ( telecare ) OR TITLE-ABS-KEY ( telemonitoring ) OR TITLE-ABS-KEY ( telehomecare ) OR TITLE-ABS-KEY ( "Remote monitoring" ) OR TITLE-ABS-KEY ( teleconsult ) OR TITLE-ABS-KEY ( "remote consult" ) ) ) ) |
| Web of Science |
1: TS = ((Alzheimer's Disease) OR dementia OR (cognitive impairment))
2: TS = (Telemedicine OR (Tele-medicine) OR Telehealth OR (Tele-health) OR Telecare OR Telemonitoring OR Telehomecare OR (Remote monitoring) OR teleconsult OR (remote consult))
#1 AND #2 |
Inclusion and Exclusion Criteria
Interventional and observational studies regarding the use of telemedicine services for patients with Alzheimer’s disease were included in the study. Studies were excluded if they were not focused on Alzheimer’s disease, along with review articles, opinion papers, editorials, letters to the editor, and the abstracts presented in a conference.
Review Process
Figure 1 shows the flow diagram of the review process. Three authors independently screened the titles and abstracts of the articles to find other relevant studies based on the inclusion and exclusion criteria. Relevant articles were selected for a full-text review, and disagreements were resolved by consensus. A data list of the selected full-text articles was generated in the Excel Spreadsheet software version 2018.
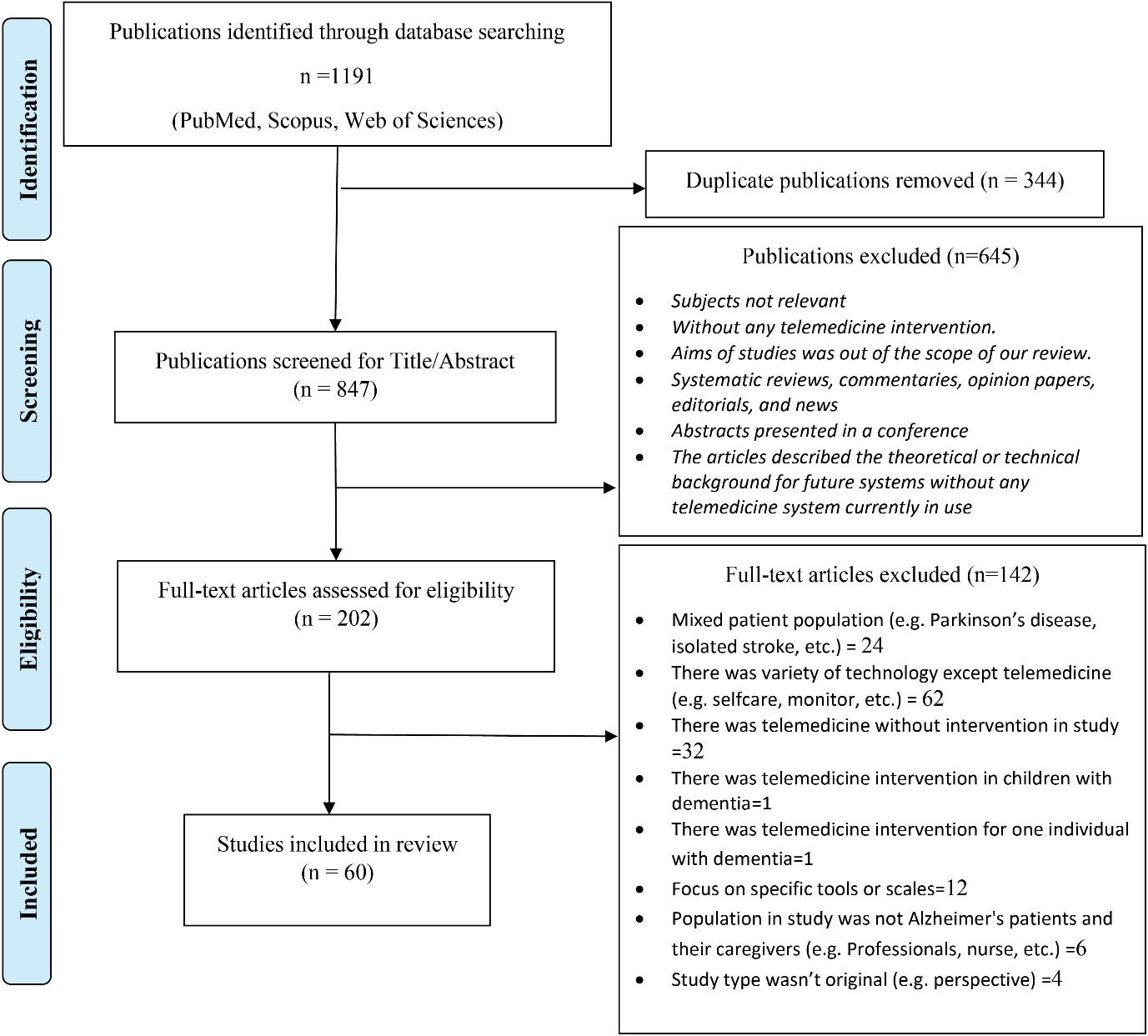
Figure 1.
Flow Diagram of Article Selection
.
Flow Diagram of Article Selection
Data Extraction and Analysis
In the present study, we identified the drivers and barriers influencing the application of telemedicine systems using qualitative content analysis in the ATLAS.ti software version 8.4.24.30 The researchers defined the drivers and barriers to telemedicine use based on the theory of planned behavior and the health belief model. The theory of planned behavior presents a model on human behavior guidance, showing the individual’s intention to perform a specific behavior under the influence of internal constraints (character, knowledge, skills and abilities, feelings) and external constraints (time, position, behavior of others) on showing a specific behavior.31 Based on the health belief model, individuals often respond well when their health is endangered (perceived susceptibility) or when a situation involves a high risk (perceived severity), and behavior change works to their benefit (perceived benefits). In addition, they attempt to eliminate issues such as high costs, waste of time, and discomfort, which may prevent healthy activities (perceived barriers), so that the individual could successfully take a health-related measure (self-efficacy).32,33 Based on this definition, drivers and barriers are divided into two internal and external sets; internal drivers refer to the positive feedback, comments, factors, and indicators that affect the behavior and motivation of the individual while using the system. External drivers include the positive feedback, opinions, factors, and indicators regarding the surrounding environment of applying the system and the system itself. On the other hand, internal barriers may refer to the negative feedback, opinions, factors, and indicators that affect the behavior and motivation of the individual while using the system, and external barriers could be considered the negative feedback, opinions, factors, and indicators regarding the surrounding environment of applying the system and the system itself.
After extracting the drivers and barriers influencing telemedicine from the full-text of the articles, several group sessions were held to discuss the discovered data and themes and reach a consensus. Moreover, the authors extracted the basic features of each study, summarized the research objectives, and described the telemedicine systems used in each study. The effectiveness of the telemedicine systems used in the studies was summarized, as well.
Results
In total, 1191 articles were retrieved, and the number was reduced to 847 articles after eliminating duplicate publications, titles, and abstracts. Finally, the full texts of 202 out of 847 articles were reviewed. Table S1 shows the data of 60 articles (Supplementary file 1).
Data of the Selected Studies
Table S1 shows the retrieved articles and their year of publication (1998–2021). Accordingly, most of the articles (n = 10; 16.7%) were conducted in 2018 and in the United States. One study was published in the form of a joint article between the United States and the United Kingdom,34 and four other papers (6.7%) were jointly performed by several countries.35-38 Among these, two cases were not included due to the multiplicity of the countries.35,36 Notably, only five articles (8.3%) were published in Asian countries (China, Taiwan, and South Korea).14,39-42
In the present study, 50, seven, and three of 60 articles were quantitative, qualitative, and mixed, respectively (Table S1). Moreover, 29 of the quantitative articles (48.3%) were randomized clinical trials. Most of the studies (n = 47; 78.3%) were performed in affiliation with an academic center. In 34 studies (56.7%), the patients or their caregivers received medical services at home. In 26 studies (43.3%), health services were provided to the patients and their caregivers at equipped hospital rooms, rural clinics, outpatient clinics, universities, and healthcare centers.
Participants
In the reviewed studies, the participants included patients with Alzheimer’s disease (60%), patients with cognitive disorders (13.3%), their caregivers (50%), and healthcare providers (5%). One of the studies recorded the number of phone calls rather than the number of participants.43 According to our findings, the target population of 24 studies (40%) was patients with Alzheimer’s disease and healthcare providers, which mainly focused on the support for caregivers. Moreover, 12 studies were similar in terms of the patients, their caregivers, and healthcare providers, and their objective was to assess the support for both groups (patients with Alzheimer’s disease and their caregivers).
Duration of the Studies
The duration of the reviewed studies differed in terms of weeks, months, and years. The shortest period was two weeks,44,45 and the longest period was 10–20 years.46 Notably, the duration of the study was not mentioned in five articles.12,37,47-49
Type of Caregivers
Two types of caregivers were mentioned in the reviewed studies (family caregiver and health staff care provider). In 60 studies, spouses (30%) and adults (19.6%) constituted the majority of the caregivers. Two studies reported the caregivers to be the family members of the patients without specifically mentioning their kinship.38,50 Assuming spouses and adult children as family members, about 60% of the caregivers of the patients with Alzheimer’s disease are their family members. Approximately 9% of caregivers were health staff care provider.
Intervention Technologies
Various technologies were applied as an intervention in the reviewed studies. More than half of the studies (n = 38; 63.3%) used a video conference technology, in which videos and images are exchanged to provide healthcare services. On the other hand, telephone-based technology was used in 13 studies (21.6%) to provide and receive healthcare services. Other technologies (such as portal, website, sensor etc.) were used in 9 studies to provide and receive healthcare services. Most of the technologies applied in the articles required an internet connection for communication (76.8%), with the exception of telephone-based interventions.
Objectives of the Studies
In total, 60 studies had mentioned the objectives, and three had different objectives.42,51,52 Ten studies assessed the feasibility of telemedicine implementation for the support of patients with Alzheimer’s disease and their caregivers. In total, 12 articles compared the effectiveness of these systems with in-person visits. In addition, most of the studies (n = 14; 23.3%) used telemedicine as a complementary technique to improve the status of patients and their caregivers. Three articles assessed and validated scales, such as the mini-mental state examination and Alzheimer’s disease assessment scale cognitive subscale, in telemedicine systems.53-55 The other studies had other objectives, such as design, implementation, follow-up, and evaluation.
Outcomes
The outcomes of the reviewed studies were divided into three categories of cost-effectiveness, patient satisfaction, and clinical outcomes. In total, 20 out of 60 articles (33.3%) addressed the cost-effectiveness of telemedicine compared to in-person visits. According to the obtained results, the cost-effectiveness of telemedicine was due to decreased commute, fuel consumption, and time to access to care. In 18 studies (32.14%), the users of telemedicine were extremely satisfied with the system. Seven studies (12.5%) also reported improved satisfaction of patients and their caregivers, and 11 studies (19.6%) showed improved satisfaction in the patients and healthcare providers after using telemedicine services (32.14%). In fact, satisfaction with telemedicine was higher in patients, their caregivers, and healthcare providers compared to traditional visits. Eight articles (14.2%) reported increased quality of life by using telemedicine. In addition, these systems were able to partially reduce general anxiety, stress, and depression, as well as the anxiety caused by the loss/injury of the patients in their caregivers through meeting some of the basic needs of the patients and their caregivers (21.42%).
Drivers
The improvement of clinical outcomes, cost-effectiveness, and patient satisfaction were among the main drivers of telemedicine use. Other drivers were also extracted from the evaluated studies. In total, 45 articles evaluated drivers (Figure 2), the most common of which were easier access to healthcare services in remote areas through telemedicine (n = 13; 23.21%), decreased commute for in-person visits/saving time (n = 12; 21.42%), and reduced burden of the disease on the caregivers, such as bathing, clothing, and taking the patient to visits (n = 10; 17.8%).
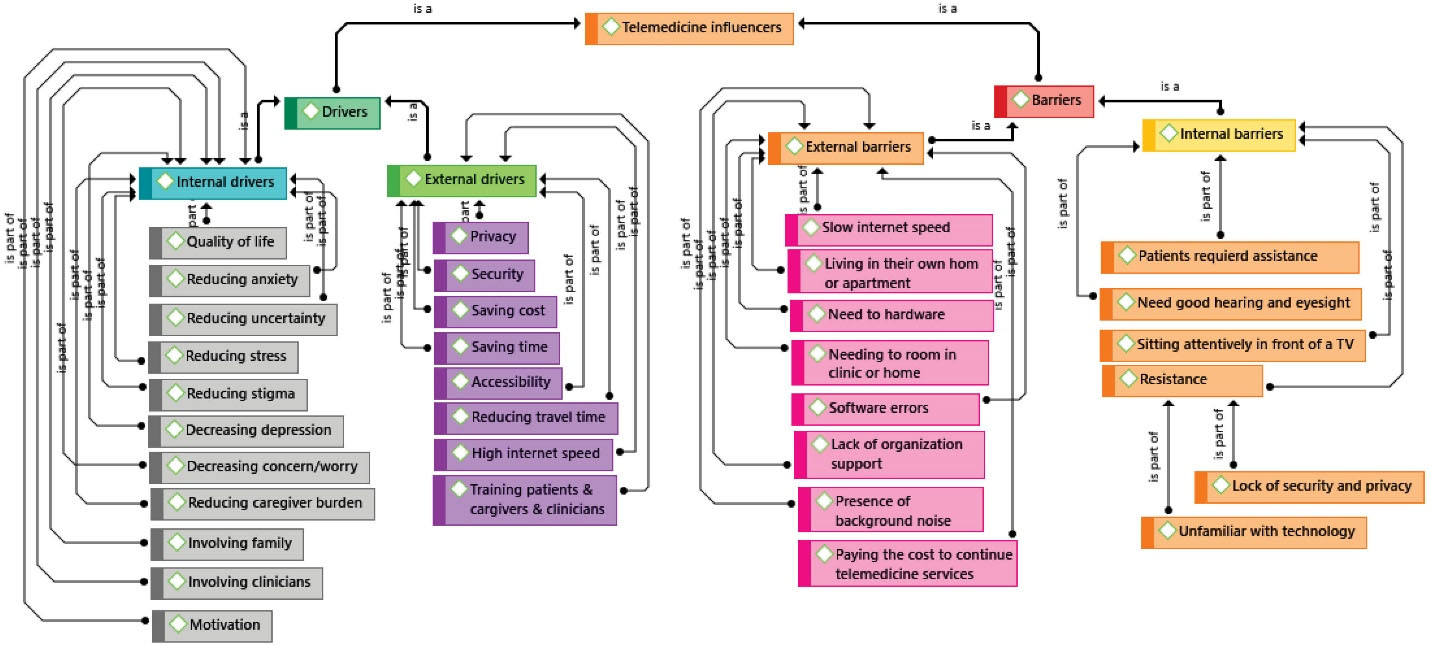
Figure 2.
Drivers and Barriers Affecting Telemedicine Systems for Patients with Alzheimer’s Disease and their Caregivers
.
Drivers and Barriers Affecting Telemedicine Systems for Patients with Alzheimer’s Disease and their Caregivers
Barriers
In 49 studies, various barriers to the use of telemedicine systems were reported (Figure 2). Some of these factors were the need to make at least one in-person visit despite the use of telemedicine services (n = 22; 39.2%), the patient’s need for at least one companion to better interact with healthcare providers during visits (n = 9; 16%), the need for high-speed internet (n = 20; 35.7%), the need for hardware (n = 21; 37.5%), and concerns about information security and privacy (n = 11, n = 8; 19.6% and 14.3%, respectively). In these studies, the Health Insurance Portability Accountability Act was used to protect the privacy and increase the security of the patients and their caregivers.
In addition to hardware barriers, which were frequently mentioned in the reviewed articles, software barriers were also reported in some studies. Software errors included problems related to sound echo or the short delay between image display/audio playback,56 noise,47,57 and technical issues.43,58 According to the results of seven studies, one of the important barriers to the successful construction of telemedicine systems was the lack of a suitable place to provide services, such as a private room in a hospital/clinic with the necessary equipment. The assessment of the articles indicated that the drivers and barriers influencing the use of telemedicine were classified as internal and external factors (Figure 2).
Discussion
In the present study, 60 studies were identified regarding the use of telemedicine designed for patients with Alzheimer’s disease and their caregivers. We mainly attempted to express the objectives, outcomes, drivers, and barriers affecting the design and implementation of telemedicine systems for further investigations. According to our data, a few systematic reviews focused on telemedicine systems for patients with Alzheimer’s disease and their caregivers, while no study assessed the drivers and barriers affecting the use of these systems.27,28,59 Notably, the systematic reviews in this regard have yielded different results. For instance, Costanzo et al only focused on the diagnostic and international outcomes of telemedicine systems in patients with Alzheimer’s disease and mild cognitive disorders up to 2018.27 According to the results of the mentioned study, telemedicine use had benefits such as remote diagnosis, facilitation of early diagnosis, decreased burden on the caregivers, and overcoming issues such as traveling long distances. Therefore, frequent use of these systems by patients could enhance their quality of life. Costanzo et al performed another study in 2014 to evaluate the impact of telemedicine use on improvement of the quality of life in patients with Alzheimer’s disease and their caregivers.28 The researcher selected articles only from the PubMed database up to 2012, and the results were indicative of the improved quality of life for the patients and their caregivers with increased use of telemedicine. Furthermore, telemedicine was reported to contribute to early diagnosis in remote areas.
In the reviewed studies, telemedicine systems were divided into two categories of home systems (simultaneous) and clinical systems (asynchronous). Home telemedicine systems encompassed online counseling, video counseling,60 and telehome monitoring.61 These systems have been reported to provide faster access to health services compared to the clinical systems. As a result, the patients and caregivers experience less stress and anxiety about the consequences of the disease and medications since they have continuous access to health services. In addition, these patients and their caregivers have been shown to have a simpler life by using telehome monitoring, while the establishment of these systems requires a high-speed internet connection.
The telemedicine systems give caregivers and healthcare providers multiple possibilities to create their own resources for the patients’ empowerment.
To date, one of the goals of technology has been to improve health care and empower and improve the conditions of various patients.62 A key approach to achieving these goals is to access/provide remote health services42 as these systems could empower individuals by increasing their participation in the treatment process.63 Given that patients with Alzheimer’s disease are mostly old, a systematic review has recently reported that telemedicine care is currently more accessible to the elderly.64 However, it is still assumed that the feasibility of this method should be further evaluated in controlled randomized clinical trials since successful results of high-quality controlled randomized trials will motivate more users.
Another quarter of the reviewed studies were classified as feasibility studies, which also compared telemedicine systems with in-person visits (controlled randomized trials). To date, several feasibility and comparative studies have focused on various diseases, including Alzheimer’s. For instance, a review conducted in 2017 showed that high-quality comparative studies have demonstrated the effectiveness of teledermatology in these patients.65 A few studies have also focused on the acceptability, effectiveness, feasibility, and economic aspects of these systems. A recent systematic review evaluated the effectiveness and economic aspects of telemedicine systems in emergency sections,66 highlighting the need for the adequate knowledge of conducting such studies. In another review (2017), telemedicine services were reported to be cost-effective in major fields of medicine, such as cardiology.67
Despite the high costs of telemedicine facilities due to the need for an internet connection or purchasing video/audio communication tools and/or sensors for disease monitoring, the application of this system could reduce commuting and waiting time for visits, while increasing its own cost-effectiveness. According to the Alzheimer’s Association Report, a minimum of three in-person visits should be made for these patients per year, which may be costly for their families.9 In Saudi Arabia, Alaboudi et al reported the need for sustainable financial support to purchase, establish, use, and maintain the complicated infrastructures required for telemedicine.68 Therefore, the cost-effectiveness of this system should be further assessed in interventional studies in more details. Moreover, patient satisfaction could affect the use of these systems. In a systematic review, patient satisfaction was reported to be the most important reason for the success of telemedicine.69 The satisfaction of patients and their caregivers with telemedicine depends on several factors.
In the current research, the influential factors in the increased satisfaction of patients and their caregivers with the telemedicine system included maintaining their privacy, reducing the stress caused by commute for in-person visits, reduction of the waiting time, increased access to care, visiting physicians while accompanied by family members, and decreased burden on the caregivers. Moreover, studies have reported other issues such as the reduction of readmission for surgery70 and contributing to the treatment plan adherence.71
Another factor that could increase the motivation to use telemedicine is improved clinical outcomes. Reduced anxiety, improved quality of life, and enhanced patient care through these systems have been mentioned in several studies. Falling has long been considered a major cause of injury and increased risk of mortality in patients with Alzheimer’s disease.72,73 Evidence attests to the concerns regarding these patients’ fall and the possibility of injury to themselves or others74 since falling is also the main cause of injury and mortality in the elderly.75,76 According to the results of our systematic review, telemedicine systems could decrease the risk of injuries in these patients, while increasing their safety and improving their quality of life and care.
In the current research, the drivers were classified as internal and external factors (Figure 2). Overall, we determined 11 internal drivers and eight external drivers regarding the implementation of telemedicine systems (Figure 2). Internal drivers could be beneficial to the users’ compatibility and acceptance of telemedicine systems by improving their quality of life and decreasing their stress and anxiety. On the other hand, external drivers could increase the effectiveness of the system by covering financial and security issues in the implementation and establishment of the systems. Evidently, the key role of these systems in improving the quality of life77 and mental health78 has been emphasized in several studies over decades. Evidence regarding the effectiveness of telemedicine systems in alleviating anxiety, depression, and stress is also consistent with this finding.79,80 Financially, the positive impact of telemedicine systems has been confirmed through saving costs and time, and the reduced number of commutes for in-person visits could also play a pivotal role in improving the quality of life of the patients. From the security perspective, health information must be protected regardless of the reason for its collection according to the HIPPA.81
While the main goal of telemedicine is to reduce barriers to the use of healthcare services, our findings indicated internal and external barriers to using telemedicine systems largely influence the users. In general, we identified four main internal barriers and eight external barriers to the use of telemedicine systems (Figure 2). The most common internal barrier was the need for at least one in-person visit in the clinic/physician office, and the most frequent external barrier was the lack of access to a high-speed internet connection. Since most of the telemedicine systems used in the reviewed studies were web-based and only a few were based on phone calls, access to a high-speed internet connection had the most significant effect on the quality of non-telephone systems. In addition to the costs of purchasing proper software and hardware, the lack of a guarantee for the security and privacy of the users largely influenced the lack of acceptance of these systems.
One of the strengths of our review study is having the maximum time interval in selecting the articles compared to similar systematic studies. In addition, a guideline or framework was presented, which contained the drivers and barriers influencing the design and implementation of telemedicine systems by researchers. One of the major limitations of the current research is the constraint in terms of selecting English articles as numerous studies in this regard have been published in non-English speaking countries, such as Korea, China, Taiwan, France, Italy, Germany, Greece, Finland, Denmark, Scotland, Rome, the Netherlands, Sweden, Iceland, and Malta. Limiting the research scope to three databases of PubMed, Scopus, and Web of Science might have led to the exclusivity of some eligible and important articles. To prevent this issue, we assessed the references of the retrieved articles, as well, in order not to eliminate relevant studies.
According to the results, it is recommended that a clear framework of the drivers and barriers affecting the use of telemedicine systems should be used by the designers and implementers of this technique for patients with Alzheimer’s disease and their caregivers. The framework could provide the users with an overview before implementing the systems, so that better decisions could be made before attempting to design and implement these systems. For instance, lack of access to a high-speed internet connection could prevent the implementation of non-telephone telemedicine systems. Moreover, studies have mostly examined the routine of telemedicine systems to show that these systems have not replaced conventional in-person systems and have only been assistive in this regard. The comparison of telehome monitoring with clinical telemedicine systems has not been apparent either. Therefore, it is recommended that further studies evaluate and compare telehome monitoring with clinical telemedicine for patients with Alzheimer’s disease and their caregivers.
Supplementary Materials
Supplementary file 1 contains Table S1.
(pdf)
Authors’ Contribution
All authors took part in the entire study and approved final manuscript. PA and KB contributed to the study design; PA, KB and HP were responsible for database search; PA and KB conducted study selection and data extraction; PA drafted the manuscript; ZN, HP and MSh critically revised manuscript for important intellectual content.
Availability of Data and Material
Our data or material may be available from corresponding author or first author upon reasonable request.
Consent for Publication
The manuscript does not contain any individual person’s data in any form.
Conflict of Interest Disclosures
No authors of this study have personal, professional, or financial conflicts of interest to declare.
Ethical Statement
This study was supported by the Student Research Committee of Kerman University of Medical Sciences (code:400000103).
References
- Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015-The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International; 2015.
- Alzheimer Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement (N Y) 2019; 15(3):321-87. doi: 10.1016/j.jalz.2019.01.010 [Crossref] [ Google Scholar]
- WHO. mhGAP: Mental Health Gap Action Programme: scaling up care for mental, neurological and substance use disorders. Geneva: World Health Organization; 2008. Available from: https://apps.who.int/iris/handle/10665/43809.
- Colucci L, Bosco M, Fasanaro AM, Gaeta GL, Ricci G, Amenta F. Alzheimer’s disease costs: what we know and what we should take into account. J Alzheimers Dis 2014; 42(4):1311-24. doi: 10.3233/JAD-131556 [Crossref] [ Google Scholar]
- Melo A. Dificuldades sentidas pelo cuidador de um doente com Alzheimer. Revisão bibliográfica; 2010.
- Chiao CY, Wu HS, Hsiao CY. Caregiver burden for informal caregivers of patients with dementia: A systematic review. Int Nurs Rev 2015; 62(3):340-50. doi: 10.1111/inr.12194 [Crossref] [ Google Scholar]
- Wimo A, Jonsson L. Cost of Illness and Burden of Dementia in Europe Prognosis to 2030. Alzheimer Europe; 2009.
- Abdolahi A, Bull MT, Darwin KC, Venkataraman V, Grana MJ, Dorsey ER. A feasibility study of conducting the Montreal Cognitive Assessment remotely in individuals with movement disorders. Health Informatics J 2016; 22(2):304-11. doi: 10.1177/1460458214556373 [Crossref] [ Google Scholar]
- Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement (N Y) 2018; 14(3):367-429. [ Google Scholar]
- Watson B, Tatangelo G, McCabe M. Depression and anxiety among partner and offspring carers of people with dementia: a systematic review. Gerontologist 2019; 59(5):e597-e610. doi: 10.1093/geront/gny049 [Crossref] [ Google Scholar]
- Bahar-Fuchs A, Webb S, Bartsch L, Clare L, Rebok G, Cherbuin N. Tailored and adaptive computerized cognitive training in older adults at risk for dementia: a randomized controlled trial. J Alzheimers Dis 2017; 60(3):889-911. doi: 10.3233/JAD-170404 [Crossref] [ Google Scholar]
- Loh PK, Donaldson M, Flicker L, Maher S, Goldswain P. Development of a telemedicine protocol for the diagnosis of Alzheimer’s disease. J Telemed Telecare 2007; 13(2):90-4. doi: 10.1258/135763307780096159 [Crossref] [ Google Scholar]
- Loh P. Telemedicine and Alzheimer’s disease from studio-based videoconferencing to mobile handheld cell phones. J Brain Dis 2009; 1:39-43. doi: 10.4137/jcnsd.s2296 [Crossref] [ Google Scholar]
- Spadaro L, Timpano F, Marino S, Bramanti P. Telemedicine and Alzheimer Disease: ICT-Based Services for People with Alzheimer Disease and their Caregivers. Telehealth Networks for Hospital Services: New Methodologies: IGI Global; 2013. p. 191-206. 10.4018/978-1-4666-2979-0.ch013.
- Wray LO, Shulan MD, Toseland RW, Freeman KE, Vásquez BE, Gao J. The effect of telephone support groups on costs of care for veterans with dementia. Gerontologist 2010; 50(5):623-31. doi: 10.1093/geront/gnq040 [Crossref] [ Google Scholar]
- Lindauer A, Croff R, Mincks K, Mattek N, Shofner S, Bouranis N. “It Took the Stress out of Getting Help”: The STAR-C-Telemedicine Mixed Methods Pilot. Care Wkly 2018; 2:7. doi: 10.14283/cw.2018.4 [Crossref] [ Google Scholar]
- Rogove HJ, McArthur D, Demaerschalk BM, Vespa PM. Barriers to telemedicine: survey of current users in acute care units. Telemed J E Health 2012; 18(1):48-53. doi: 10.1089/tmj.2011.0071 [Crossref] [ Google Scholar]
- Council on Competitiveness. Highway to health: transforming US health care in the information age. Washington, DC: Council on Competitiveness; 1996.
- Allaire P, Young J, Vest C, eds. Breaking the barriers to the national information infrastructure. A conference report by the Council on Competitiveness. Washington DC; 1994.
- Abbate S, Avvenuti M, Light J. Usability study of a wireless monitoring system among Alzheimer’s disease elderly population. Int J Telemed Appl 2014; 9(3):350-66. doi: 10.1155/2014/617495 [Crossref] [ Google Scholar]
- Perednia DA, Allen A. Telemedicine technology and clinical applications. JAMA 1995; 273(6):483-8. [ Google Scholar]
- Alexandru A, Ianculescu M. Enabling assistive technologies to shape the future of the intensive senior-centred care: A case study approach. Studies in Informatics and Control 2017; 26(3):343-52. doi: 10.24846/v26i3y201710 [Crossref] [ Google Scholar]
- WHO. Telemedicine: opportunities and developments in member states. Report on the second global survey on eHealth: World Health Organization; 2010. Available from: https://apps.who.int/iris/handle/10665/44497.
- MacFarlane A, Murphy AW, Clerkin P. Telemedicine services in the Republic of Ireland: an evolving policy context. Health Policy 2006; 76(3):245-58. doi: 10.1016/j.healthpol.2005.06.006 [Crossref] [ Google Scholar]
- Zanaboni P, Wootton R. Adoption of telemedicine: from pilot stage to routine delivery. BMC Med Inform Decis Mak 2012; 12:1. doi: 10.1186/1472-6947-12-1 [Crossref] [ Google Scholar]
- Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare 2018; 24(1):4-12. doi: 10.1177/1357633X16674087 [Crossref] [ Google Scholar]
- Costanzo MC, Arcidiacono C, Rodolico A, Panebianco M, Aguglia E, Signorelli MS. Diagnostic and interventional implications of telemedicine in Alzheimer’s disease and mild cognitive impairment: a literature review. Int J Geriatr Psychiatry 2020; 35(1):12-28. doi: 10.1002/gps.5219 [Crossref] [ Google Scholar]
- Costanzo M, Signorelli M, Aguglia E. EPA-0715–telemedicine and Alzheimer: a systematic review. European Psychiatry 2014; 29(S1):1-20. doi: 10.1016/S0924-9338(14)78073-3 [Crossref] [ Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7):e1000097. doi: 10.1136/bmj.b2535 [Crossref] [ Google Scholar]
- Wildemuth BM. Applications of social research methods to questions in information and library science. ABC-CLIO; 2016.
- Ajzen I. From intentions to actions: A theory of planned behavior. In: Kuhl J, Beckmann J, eds. Action Control. Berlin, Heidelber: Springer; 1985. p. 11-39. 10.1007/978-3-642-69746-3_2.
- Andersen RM. National health surveys and the behavioral model of health services use. Med Care. 2008:647-53. 10.1097/MLR.0b013e31817a835d.
- Andersen RM. Revisiting the behavioral model and access to medical care: does it matter?. J Health Soc Behav 1995; 36(1):1-10. [ Google Scholar]
- Pakrasi S, Burmeister O, Coppola J, McCallum T, Loeb G. Ethical telehealth design for users with dementia. Gerontechnology 2015; 13(4):383-387. doi: 10.4017/gt.2015.13.4.002.00 [Crossref] [ Google Scholar]
- Hattink B, Meiland F, van der Roest H, Kevern P, Abiuso F, Bengtsson J. Web-based STAR E-learning course increases empathy and understanding in dementia caregivers: results from a randomized controlled trial in the Netherlands and the United Kingdom. J Med Internet Res 2015; 17(10):e241. doi: 10.2196/jmir.4025 [Crossref] [ Google Scholar]
- Mitseva A, Peterson CB, Karamberi C, Oikonomou LC, Ballis AV, Giannakakos C. Gerontechnology: providing a helping hand when caring for cognitively impaired older adults—intermediate results from a controlled study on the satisfaction and acceptance of informal caregivers. Curr Gerontol Geriatr Res 2012; 19(1):200-229. doi: 10.1155/2012/401705 [Crossref] [ Google Scholar]
- Bowes A, Dawson A, McCabe L. RemoDem: Delivering support for people with dementia in remote areas. Dementia 2018; 17(3):297-314. doi: 10.1177/1471301216643848 [Crossref] [ Google Scholar]
- Harvey RJ, Roques PK, Fox NC, Rossor MN. CANDID—counselling and diagnosis in dementia: a national telemedicine service supporting the care of younger patients with dementia. Int J Geriatr Psychiatry 1998; 13(6):381-8. doi: 10.1002/(sici)1099-1166 [Crossref] [ Google Scholar]
- Cheong C-K, Lim K-H, Jang J-W, Jhoo JH. The effect of telemedicine on the duration of treatment in dementia patients. J Telemed Telecare 2015; 21(4):214-8. doi: 10.1177/1357633X14566571 [Crossref] [ Google Scholar]
- Lee JH, Kim JH, Jhoo JH, Lee KU, Kim KW, Lee DY. A telemedicine system as a care modality for dementia patients in Korea. Alzheimer Dis Assoc Disord 2000; 14(2):94-101. doi: 10.1097/00002093-200004000-00007 [Crossref] [ Google Scholar]
- Poon P, Hui E, Dai D, Kwok T, Woo J. Cognitive intervention for community‐dwelling older persons with memory problems: telemedicine versus face‐to‐face treatment. Int J Geriatr Psychiatry 2005; 20(3):285-6. doi: 10.1002/gps.1282 [Crossref] [ Google Scholar]
- Chou H-K, Yan S-H, Lin I-C, Tsai M-T, Chen C-C, Woung L-C. A pilot study of the telecare medical support system as an intervention in dementia care: the views and experiences of primary caregivers. Journal of Nursing Research 2012; 20(3):169-80. doi: 10.1097/jnr.0b013e318263d916 [Crossref] [ Google Scholar]
- Smith GE, Lunde AM, Hathaway JC, Vickers KS. Telehealth home monitoring of solitary persons with mild dementia. Am J Alzheimers Dis Other Demen 2007; 22(1):20-26. doi: 10.1177/1533317506295888 [Crossref] [ Google Scholar]
- Harrell KM, Wilkins SS, Connor MK, Chodosh J. Telemedicine and the evaluation of cognitive impairment: The additive value of neuropsychological assessment. J Am Med Dir Assoc 2014; 15(8):600-6. doi: 10.1016/j.jamda.2014.04.015 [Crossref] [ Google Scholar]
- Lindauer A, Seelye A, Lyons B, Dodge HH, Mattek N, Mincks K. Dementia care comes home: patient and caregiver assessment via telemedicine. Gerontologist 2017; 57(5):e85-e93. doi: 10.1093/geront/gnw206 [Crossref] [ Google Scholar]
- Parikh M, Grosch MC, Graham LL, Hynan LS, Weiner M, Shore JH. Consumer acceptability of brief videoconference-based neuropsychological assessment in older individuals with and without cognitive impairment. Clin Neuropsychol 2013; 27(5):808-17. doi: 10.1080/13854046.2013.791723 [Crossref] [ Google Scholar]
- Vestal L, Smith-Olinde L, Hicks G, Hutton T, Hart Jr J. Efficacy of language assessment in Alzheimer’s disease: comparing in-person examination and telemedicine. Clin Interv Aging 2006; 1(4):467. doi: 10.2147/ciia.2006.1.4.467 [Crossref] [ Google Scholar]
- Cullum CM, Hynan L, Grosch M, Parikh M, Weiner M. Teleneuropsychology: Evidence for video teleconference-based neuropsychological assessment. J Int Neuropsychol Soc 2014; 20(10):1028-33. doi: 10.1017/S1355617714000873 [Crossref] [ Google Scholar]
- Barton C, Morris R, Rothlind J, Yaffe K. Video-telemedicine in a memory disorders clinic: evaluation and management of rural elders with cognitive impairment. Telemed J E Health 2011; 17(10):789-93. doi: 10.1089/tmj.2011.0083 [Crossref] [ Google Scholar]
- Powers JS, Buckner J. Reaching out to rural caregivers and veterans with dementia utilizing clinical video-telehealth. Geriatrics 2018; 3(2):29. doi: 10.3390/geriatrics3020029 [Crossref] [ Google Scholar]
- Dang S, Gomez-Orozco CA, van Zuilen MH, Levis S. Providing dementia consultations to veterans using clinical video telehealth: results from a clinical demonstration project. Telemed J E Health 2018; 24(3):203-9. doi: 10.1089/tmj.2017.0089 [Crossref] [ Google Scholar]
- Lindauer A, McKenzie G, LaFazia D, McNeill L, Mincks K, Spoden N. Using technology to facilitate fidelity assessments: The Tele-STAR caregiver intervention. J Med Internet Res 2019; 21(5):e13599. doi: 10.2196/13599 [Crossref] [ Google Scholar]
- Martin-Khan M, Flicker L, Wootton R, Loh P-K, Edwards H, Varghese P, et al. The diagnostic accuracy of telegeriatrics for the diagnosis of dementia via video conferencing. J Am Med Dir Assoc 2012;13(5):487. e19-. e24. 10.1016/j.jamda.2012.03.004.
- Mavandadi S, Wray LO, Toseland RW. Measuring Self-Appraised Changes following Participation in an Intervention for Caregivers of Individuals with Dementia. J Gerontol Soc Work 2019; 62(3):324-37. doi: 10.1080/01634372.2018.1556767 [Crossref] [ Google Scholar]
- Carotenuto A, Rea R, Traini E, Ricci G, Fasanaro AM, Amenta F. Cognitive assessment of patients with Alzheimer’s disease by telemedicine: pilot study. JMIR Mental Health 2018; 5(2):e8097. doi: 10.2196/mental.8097 [Crossref] [ Google Scholar]
- Shores MM, Ryan‐Dykes P, Williams RM, Mamerto B, Sadak T, Pascualy M. Identifying undiagnosed dementia in residential care veterans: comparing telemedicine to in‐person clinical examination. Int J Geriatr Psychiatry 2004; 19(2):101-8. doi: 10.1002/gps.1029 [Crossref] [ Google Scholar]
- O’Connell ME, Crossley M, Cammer A, Morgan D, Allingham W, Cheavins B. Development and evaluation of a telehealth videoconferenced support group for rural spouses of individuals diagnosed with atypical early-onset dementias. Dementia 2014; 13(3):382-95. doi: 10.1177/1471301212474143 [Crossref] [ Google Scholar]
- Azad N, Amos S, Milne K, Power B. Telemedicine in a rural memory disorder clinic—remote management of patients with dementia. Canadian Geriatrics Journal 2012; 15(4):96. doi: 10.5770/cgj.15.28 [Crossref] [ Google Scholar]
- Ferreira Santana R, Vaqueiro Dantas R, da Silva Soares T, Melo Delphino T, Serra Hercules AB, Teixeira Leite Junior HM. Telecare to elderly people with alzheimer and their caregivers: systematic review. Ciencia, Cuidado e Saude 2018;17(4). 10.4025/cienccuidsaude.v17i4.41653.
- Westra I, Niessen F. Implementing real-time video consultation in plastic surgery. Aesthetic plastic surgery 2015; 39(5):783-90. doi: 10.1007/s00266-015-0526-4 [Crossref] [ Google Scholar]
- Woodend AK, Sherrard H, Fraser M, Stuewe L, Cheung T, Struthers C. Telehome monitoring in patients with cardiac disease who are at high risk of readmission. Heart Lung 2008; 37(1):36-45. doi: 10.1016/j.hrtlng.2007.04.004 [Crossref] [ Google Scholar]
- Mahoney DF, Tarlow BJ, Jones RN. Effects of an automated telephone support system on caregiver burden and anxiety: findings from the REACH for TLC intervention study. Gerontologist 2003; 43(4):556-67. doi: 10.1093/geront/43.4.556 [Crossref] [ Google Scholar]
- Martin S, Augusto JC, McCullagh P, Carswell W, Zheng H, Wang H. Participatory research to design a novel telehealth system to support the night-time needs of people with dementia: NOCTURNAL. Int J Environ Res Public Health 2013; 10(12):6764-82. doi: 10.3390/ijerph10126764 [Crossref] [ Google Scholar]
- Tchalla AE, Lachal F, Cardinaud N, Saulnier I, Rialle V, Preux P-M. Preventing and managing indoor falls with home-based technologies in mild and moderate Alzheimer’s disease patients: pilot study in a community dwelling. Dement Geriatr Cogn Disord 2013; 36(3-4):251-61. doi: 10.1159/000351863 [Crossref] [ Google Scholar]
- Schaller S, Marinova-Schmidt V, Gobin J, Criegee-Rieck M, Griebel L, Engel S. Tailored e-Health services for the dementia care setting: a pilot study of ‘eHealthMonitor’. BMC Med Inform Decis Mak 2015; 15(1):1-9. doi: 10.1186/s12911-015-0182-2 [Crossref] [ Google Scholar]
- Mavandadi S, Wray LO, DiFilippo S, Streim J, Oslin D. Evaluation of a telephone-delivered, community-based collaborative care management program for caregivers of older adults with dementia. Am J Geriatr Psychiatry 2017; 25(9):1019-28. doi: 10.1016/j.jagp.2017.03.015 [Crossref] [ Google Scholar]
- Chang W, Homer M, Rossi MI. Use of Clinical Video Telehealth as a Tool for Optimizing Medications for Rural Older Veterans with Dementia. Geriatrics (Basel) 2018;3(3). 10.3390/geriatrics3030044.
- Wadsworth HE, Dhima K, Womack KB, Hart J Jr, Weiner MF, Hynan LS. Validity of teleneuropsychological assessment in older patients with cognitive disorders. Arch Clin Neuropsychol 2018; 33(8):1040-5. doi: 10.1093/arclin/acx140 [Crossref] [ Google Scholar]
- Töpfer NF, Wilz G. Tele TAnDem increases the psychosocial resource utilization of dementia caregivers. GeroPsych 2018; 31(4):173-183. doi: 10.1024/1662-9647/a000197 [Crossref] [ Google Scholar]
- Williams KN, Perkhounkova Y, Shaw CA, Hein M, Vidoni ED, Coleman CK. Supporting family caregivers with technology for dementia home care: A randomized controlled trial. Innov Aging 2019; 3(3):igz037. doi: 10.1093/geroni/igz037 [Crossref] [ Google Scholar]
- AHA. Most Wired: The Big Payback-2001 survey shows a healthy return on investment for info tech. AHA; 2001.
- Calvillo J, Roman I, Roa LM. How technology is empowering patients? A literature review. Health Expect 2015; 18(5):643-52. doi: 10.1111/hex.12089 [Crossref] [ Google Scholar]
- Nielsen MK, Johannessen H. Patient empowerment and involvement in telemedicine. J Nurs Educ Pract 2019; 9(8):54-8. doi: 10.5430/jnep.v9n8p54 [Crossref] [ Google Scholar]
- Batsis JA, DiMilia PR, Seo LM, Fortuna KL, Kennedy MA, Blunt HB. Effectiveness of ambulatory telemedicine care in older adults: a systematic review. J Am Geriatr Soc 2019; 67(8):1737-49. doi: 10.1111/jgs.15959 [Crossref] [ Google Scholar]
- Piga M, Cangemi I, Mathieu A, Cauli A, editors. Telemedicine for patients with rheumatic diseases: systematic review and proposal for research agenda. Semin Arthritis Rheum 2017;47(1):121-128: 10.1016/j.semarthrit.2017.03.014.
- Tsou C, Robinson S, Boyd J, Jamieson A, Blakeman R, Bosich K. Effectiveness and cost-effectiveness of telehealth in rural and remote emergency departments: a systematic review protocol. Syst Rev 2020; 9(1):1-6. doi: 10.1186/s13643-020-01349-y [Crossref] [ Google Scholar]
- Delgoshaei B, Mobinizadeh M, Mojdekar R, Afzal E, Arabloo J, Mohamadi E. Telemedicine: A systematic review of economic evaluations. Med J Islam Repub Iran 2017; 31:113. doi: 10.14196/mjiri.31.113 [Crossref] [ Google Scholar]
- Alaboudi A, Atkins A, Sharp B, Balkhair A, Alzahrani M, Sunbul T. Barriers and challenges in adopting Saudi telemedicine network: The perceptions of decision makers of healthcare facilities in Saudi Arabia. J Infect Public Health 2016; 9(6):725-33. doi: 10.1016/j.jiph.2016.09.001 [Crossref] [ Google Scholar]
- Granja C, Janssen W, Johansen MA. Factors determining the success and failure of eHealth interventions: systematic review of the literature. J Med Internet Res 2018; 20(5):e10235. doi: 10.2196/10235 [Crossref] [ Google Scholar]
- Prabhu KL, Cleghorn MC, Elnahas A, Tse A, Maeda A, Quereshy FA. Is quality important to our patients? The relationship between surgical outcomes and patient satisfaction. BMJ Qual Saf 2018; 27(1):48-52. doi: 10.1136/bmjqs-2017-007071 [Crossref] [ Google Scholar]
- Zolnierek KBH, DiMatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Medical care 2009; 47(8):826. doi: 10.1097/MLR.0b013e31819a5acc [Crossref] [ Google Scholar]