Arch Iran Med. 28(2):100-106.
doi: 10.34172/aim.33457
Original Article
Predictors of 12-Month Recurrence of Hemoptysis after Bronchial Artery Embolization
Sareh Sadidi Conceptualization, Data curation, Investigation, Validation, Writing – review & editing, 1, * 
Farzin Roozafzai Formal analysis, Writing – original draft, Writing – review & editing, 2 
Sirous Nekooei Investigation, Validation, Writing – review & editing, 1 
Lida Jarahi Methodology, Writing – review & editing, 3 
Farzaneh Khoroushi Conceptualization, Investigation, Supervision, Validation, Writing – review & editing, 1 
Author information:
1Department of Radiology, Mashhad University of Medical Sciences, Mashhad, Iran
2Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
3Department of Community Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Abstract
Background:
Despite the high success rate of bronchial artery embolization (BAE), hemoptysis probably recurs. This study investigated risk factors of 12-month hemoptysis recurrence after BAE in an Iranian population.
Methods:
In this prospective cohort, we followed up 101 patients for 12 months after BAE. Outcome of interest was recurrence of hemoptysis. Target arteries were super-selectively catheterized and embolized with non-spherical polyvinyl alcohol particles (150–700 µm). Success of BAE was confirmed using post-BAE angiography. Independent t-test, and chi-square and Fisher’s exact test were used to compare variables between "recurrence" and "non-recurrence" groups. We investigated predictors of recurrent hemoptysis through univariate and multivariate logistic regression modeling. We analyzed receiver operating characteristic curve to find the optimal cutoff point for continuous risk factors. Recurrence-free rates stratified by risk factors were plotted against time using the Kaplan-Meier method.
Results:
BAE was immediately successful in all patients. During the 12-month follow-up, hemoptysis recurred in 13.9% (95% CI: 8.2–21.6) of participants. Mean (±standard deviation) recurrence-free time was 6.9 (±3.3) months. Lung destruction (OR=5.40 [95% CI: 1.41–20.58], P value=0.013) and arterial diameter≥2 mm (12.51 [1.51–103.59], P value=0.019) were independent predictors of 12-month hemoptysis recurrence.
Conclusion:
Patients with destroyed lungs and embolized arteries wider than 2.0 mm are at higher risk of hemoptysis recurrence in the first year after BAE.
Keywords: Bronchial artery embolization, Hemoptysis, Recurrence, Risk factor
Copyright and License Information
© 2025 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Sadidi S, Roozafzai F, Nekooei S, Jarahi L, Khoroushi F. Predictors of 12-month recurrence of hemoptysis after bronchial artery embolization. Arch Iran Med. 2025;28(2):100-106. doi: 10.34172/aim.33457
Introduction
Hemoptysis, expectoration of blood from the respiratory system, is a common and potentially life-threatening symptom.1-3 The etiology of hemoptysis varies regionally: the most common causes are tuberculosis (TB) and bronchiectasis in Eastern countries, and lung cancer in Western countries.3-6 Bronchiectasis is a chronic respiratory disease with an increasing worldwide prevalence.7 Unfortunately, no data on the epidemiology of bronchiectasis is available from regions such as the Middle East.8 Neglected or incompletely treated pulmonary infections, such as TB, are the most common etiology of bronchiectasis in low-and-middle-income countries.9 TB is intermediately prevalent in Iran, particularly in eastern provinces neighboring Afghanistan and Pakistan where TB is highly prevalent and incident.10-12 Social determinants of health such as low socioeconomic status, cultural stigma, illiteracy, or limited access to diagnostic and therapeutic facilities, might make the population of those eastern provinces leave the hemoptysis untreated.13-15 The mortality rate of untreated or conservatively managed massive hemoptysis ( > 300 mL/d) is greater than 50%.2-5,16,17
Bronchial arteries are the most common anatomic source of hemoptysis.1,2,18 Bronchial artery embolization (BAE) is recommended as the first-line treatment of hemoptysis, particularly in emergency settings, and high-risk or inoperable patients.1 Advances in BAE techniques, such as super-selective coaxial microcatheterization, particulate embolization, and pre- and post-embolization angiography, have led to more effective control of hemoptysis.16,19 Despite high immediate clinical success rate, defined as complete cessation of hemoptysis after BAE, recurrence rate remains high and up to 57% of patients might experience recurrent hemoptysis after BAE.4 Incomplete BAE, bleeding from non-bronchial arteries, parenchymal lung destruction, size of embolized arteries, recanalization, and neovascularization, have been previously reported as predictors of hemoptysis recurrence.1,4,16,20-24
The high recurrence rate necessitates understanding of risk factors, tailoring treatment plans, and optimizing hemoptysis management strategies. We aimed to investigate risk factors predicting the recurrence of hemoptysis after BAE in a population of north-eastern Iranians whom the well-known etiologies of hemoptysis, such as TB and bronchiectasis, might prevalently affect.
Materials and Methods
Patients and Design
In this prospective observation, we enrolled all consecutive patients who underwent BAE for hemoptysis in two tertiary-care university hospitals in Mashhad, Iran, between 2017 and 2022. We consequently followed up the participants for 12 months looking for recurrence of hemoptysis after BAE. Subjects would be excluded if they: disagreed to participate; were younger than 18 years; had missing data or imaging; withdrew; or were lost to follow-up.
Assuming a hemoptysis recurrence rate (p) of 10%,1 a margin of error (δ) of 6%, type I error (α) of 5%, and an expected dropout rate of 30%, an approximate size (N) of 127 subjects for the sample population was calculated using the following formula:
Baseline Data
In the enrollment phase (2017–2022), we interviewed the participants, reviewed medical documents and radiologic images, and collected baseline data regarding demographics (age, sex, tobacco smoking), etiologic disease (TB, cancer, chronic obstructive pulmonary disease, and other etiologies), paraclinical assessments (prothrombin time and partial thromboplastin time), and radiologic findings. Contrast-enhanced thoracic computed tomography (CT) and CT-angiography findings included location, number, and maximum diameter of target arteries, and presence of bronchiectasis, emphysema, honeycombing, consolidation, mass, cavity, collapse, fibrosis, and destructive changes in lungs. Lung destruction was defined as diffuse parenchymal destruction or volume loss in at least one lobe.21
CT-angiography
Before BAE, CT-angiography was performed using NeuViz 16-slice CT scanner (Neusoft Corp., Shenyang, China) with 2.0 cc/kg of Visipaque (Iodixanol 320 mgI/mL; GE Healthcare, Chicago, IL, USA) injected into the antecubital vein at a speed of 4.0 mL/s. Then, the data were transferred to a post-processing workstation, reconstructed at 2.0 mm section thickness, and examined by two interventional radiologists. The CT scanner used in this study is calibrated annually by Parsian Radiation Dosimetry Services Company, Tehran, Iran. In the last quality control tests (2023, reference number: LQF-510-08), the rate of leakage/scatter of dye ( < 1 mGy/h) and the precision of CT numbers (error < 5 Hounsfield unit) were acceptable.
Bronchial Artery Embolization
Two experienced interventional radiologists performed all procedures via a transfemoral approach under local anesthesia using 4-F or 5-F guide catheters (Merit Medical, South Jordan, UT, USA). To confirm the location of bleeding, a flush descending thoracic aortogram, opacifying both bronchial and non-bronchial systemic arteries, was acquired at the beginning of BAE. This non-selective arteriography was followed by super-selective catheterization of target arteries introducing and carefully advancing a 2.9-F coaxial microcatheter (Merit Medical, South Jordan, UT, USA), to avoid non-target embolization of side branches, such as anterior spinal artery. Non-spherical polyvinyl alcohol (PVA) particles (Merit Medical, South Jordan, UT, USA), 150–700 microns in size, were injected until the proximal portion of the artery was occluded on angiography. All abnormal vessels supplying the area of interest were embolized if technically possible. No patient underwent coil embolization in our cohort. A successful BAE would significantly reduce and cease hemoptysis, and would be confirmed through a post-BAE angiography showing no blushing or distal vascular signs (technical success), with no recurrence of hemoptysis within the same admission (clinical success).3,25
Outcome of Interest
Recurrence of hemoptysis was defined as clinically significant hemoptysis ( ≥ 30 mL/d) occurring after clinical success and requiring clinical management.21
Follow-up Data
After 12 months, we actively followed-up each participant through phone calls and inquired about recurrent hemoptysis or cause-specific death, and their pertinent dates. In case of death, we would ask the participant’s family members, care-givers, or disease registries to report the cause of death and recurrence of hemoptysis, and provide the available medical documents (e.g. death certificate, or autopsy report).
Endpoints
Endpoints of the study were death, withdrawal, and loss to follow-up. Participants who did not answer three consecutive calls in a week would be considered lost.
Statistical Analysis
According to the follow-up data, participants were divided into recurrence and non-recurrence groups. We used descriptive statistics to report categorical variables (frequency distributions) and continuous variables (mean and standard deviation [SD]). We respectively used independent t-test, and chi-square and Fisher’s exact test, to compare continuous and categorical variables between recurrence and non-recurrence groups. We investigated variables predicting recurrent hemoptysis through univariate and multivariate Cox logistic regression modeling. Significant univariate predictors were inserted in the multivariate models with the backward selection method. Models were summarized in terms of odds ratio (OR) with 95% confidence interval (CI). We also plotted receiver operating characteristic (ROC) curve and used the Youden index to determine the optimal cutoff point for continuous risk factors. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, positive likelihood ratio (LR + ), negative likelihood ratio (LR-), and diagnostic odds ratio (DOR) were calculated for the selected cutoff points. We used the Kaplan-Meier method to plot recurrence-free probabilities against time in different categories of risk factors, and compared the categories using the Cox-Mantel log-rank test. We did not investigate potential confounding factors, such as socioeconomic status, comorbidities, or medications. The data were analyzed using IBM SPSS v.25 (IBM corp., Chicago, IL, USA). Level of significance was 0.05.
Results
Of 127 recruited patients (76 men and 51 women), we excluded three unwilling subjects and nine with missing data. We collected the baseline data of 115 participants in the enrollment and followed up the participants for 12 months. Attrition of the cohort included 14 participants who did not answer the follow-up phone calls. We finally analyzed the data of 101 participants comprising 64 men (63.4%) and 37 women (36.6%). Figure 1 shows the study flowchart.
Figure 1.
Schematic Flowchart of the Study. BAE: bronchial artery embolization
.
Schematic Flowchart of the Study. BAE: bronchial artery embolization
Hemoptysis was successfully controlled immediately after BAE in all patients. During the 12-month follow-up, hemoptysis recurred in 14 cases (recurrence group; 13.9% [95% CI: 8.2–21.6]) with a mean ( ± SD) recurrence-free duration of 6.9 ( ± 3.3) months. The other 87 participants were recurrence-free after 12 months (non-recurrence group; 86.1% [95% CI: 78.4–91.8]). Cumulative hemoptysis recurrence-free rates at the first, third, sixth, and ninth months after BAE were 1.00, 0.97, 0.93, and 0.91, respectively.
There was no significant difference in sex distribution between the recurrence and non-recurrence groups (P value = 1.000). The mean ( ± SD) age was 61.0 ( ± 15.9) years, showing no significant difference between the recurrence and non-recurrence groups (Table 1).
Table 1.
Comparison of Baseline Characteristics in Recurrence and Non-recurrence Groups of Patients with Hemoptysis Treated by Bronchial Artery Embolization
|
Characteristics
|
Total (n=101)
|
Recurrent Hemoptysis
|
Difference (95% CI)c
|
P
Value*
|
|
Yes (n=14)
|
No (n=87)
|
|
Mean±Standard Deviation
|
| Age (y) |
61.0 ± 15.9 |
65.0 ± 16.2 |
60.4 ± 15.9 |
4.5 (-4.5–13.6) |
0.327 |
| Diameter of target artery (mm) |
2.0 ± 0.5 |
2.2 ± 0.3 |
1.9 ± 0.5 |
0.31 (0.01–0.60) |
0.040 |
| PT (second) |
13.6 ± 2.4 |
13.6 ± 1.8 |
13.7 ± 2.5 |
-0.1 (-1.5–1.2) |
0.870 |
| PTT (second) |
29.9 ± 16.6 |
25.3 ± 4.0 |
30.7 ± 17.8 |
-5.3 (-14.9–4.13) |
0.264 |
|
|
Count (Percent)a
|
|
P
Value**
|
| Age ≥ 65 years |
47 (46.5) |
8 (57.1) |
39 (44.8) |
12.3 % (-15.6–40.2) |
0.406 |
| Diameter of target artery ≥ 2 mm |
58 (57.4) |
13 (92.9) |
45 (51.7) |
41.2 % (24.0–58.2) |
0.003 |
| Male sex |
64 (63.4) |
9 (64.3) |
55 (63.2) |
1.1 % (-25.9–28.1) |
1.000 |
| Tobacco smoking |
27 (26.7) |
6 (42.9) |
21 (24.1) |
18.8 % (-8.6–46.2) |
0.192 |
| TB |
31 (30.7) |
7 (50.0) |
24 (27.6) |
22.4 % (-5.4–50.2) |
0.120 |
| Cancer b |
11 (10.9) |
3 (21.4) |
8 (9.2) |
12.2 % (-10.1–34.5) |
0.178 |
| Lung cancer |
6 (5.9) |
2 (14.3) |
4 (4.6) |
9.7 % (-9.1–28.5) |
0.193 |
| COPD |
7 (6.9) |
0 (0.0) |
7 (8.0) |
-8.0 % (-13.7–-2.3) |
0.589 |
| Emphysema |
26 (25.7) |
2 (14.3) |
24 (27.6) |
13.3 % (-33.9–7.3) |
0.510 |
| Bronchiectasis |
58 (57.4) |
11 (78.6) |
47 (54.0) |
24.6 % (0.7–48.5) |
0.144 |
| Honeycombing |
2 (1.9) |
1 (7.1) |
1 (1.1) |
6.0 % (-7.6–19.6) |
0.259 |
| Consolidation |
47 (46.5) |
8 (57.1) |
39 (44.8) |
12.3 % (-15.6–40.2) |
0.406 |
| Collapse |
15 (14.9) |
4 (28.6) |
11 (12.6) |
16.0 % (-8.6–40.6) |
0.216 |
| Fibrotic changes |
44 (43.6) |
10 (71.4) |
34 (39.1) |
32.3 % (6.5–58.1) |
0.039 |
| Destructive changes |
17 (16.8) |
6 (42.9) |
11 (12.6) |
30.3 % (3.3–57.0) |
0.012 |
| Cavity |
33 (32.7) |
7 (50.0) |
26 (29.9) |
20.1 % (-7.8–48.0) |
0.217 |
| Mass |
13 (12.9) |
3 (21.4) |
10 (11.5) |
9.9 % (-12.6–32.4) |
0.383 |
| Size of PVA particle |
150–300 µm |
17 (16.8) |
3 (21.4) |
14 (16.1) |
5.3 % (-17.5–28.1) |
0.849 |
| 300–500 µm |
60 (59.4) |
8 (57.1) |
52 (59.8) |
-2.7 % (-30.6–25.2) |
| 500–700 µm |
24 (23.8) |
3 (21.4) |
21 (24.1) |
-2.7 % (-25.9–20.5) |
| Embolized artery |
Bronchial |
38 (37.6) |
3 (21.4) |
35 (40.2) |
-18.8 % (-42.6–5.0) |
0.155 |
| Non-bronchial |
9 (8.9) |
0 (0.0) |
9 (10.3) |
-10.3 % (-16.6–-3.9) |
| Both |
54 (53.5) |
11 (78.6) |
43 (49.4) |
29.2 % (5.2–53.1) |
| Number of embolized arteries |
1 artery |
28 (27.7) |
1 (7.1) |
27 (31.0) |
-23.9 % (-40.5–-7.3) |
0.074 |
| 2 arteries |
44 (43.6) |
6 (42.9) |
38 (43.7) |
-0.8 % (-28.7–27.1) |
| ≥ 3 arteries |
29 (28.7) |
7 (50.0) |
22 (25.3) |
24.7 % (-3.0–52.4) |
CI: confidence interval, COPD: chronic obstructive pulmonary disease, n: count, PT: prothrombin time, PTT: partial thromboplastin time, PVA: polyvinyl alcohol, TB: tuberculosis.
*Independent samples t-test; level of significance < 0.05
**Fisher’s exact test; level of significance < 0.05
aNumbers represent frequency distributions in the column.
bIncluding lung cancer, metastatic cancer of other origins, and lymphoma.
cCalculated by subtracting values in “No” column from values in “Yes” column.
As represented in Table 1, significant differences were found in distribution of destructive and fibrotic changes, and maximum diameter of embolized artery between the recurrence and non-recurrence groups. Lung destruction (42.9% [95% CI: 20.3–68.1]) and fibrotic changes (71.4% [45.5–89.5]) were more prevalent in the recurrence group than in the non-recurrence group (12.6% [6.9–20.8] and 39.1% [29.3–49.6], respectively); and, the diameter of embolized artery was 0.31 mm (95% CI: 0.01–0.60) wider in the recurrence group.
Based on the ROC curve (Figure 2), we selected 2.0 mm (Youden index = 0.412) as the cutoff for maximum arterial diameter for discriminating the recurrence. The sensitivity, specificity, PPV, NPV, accuracy, LR + , LR-, and DOR for the selected cutoff point were 92.8%, 48.2% 22.4%, 97.6%, 54.4%, 1.79, 0.14, and 12.13, respectively. Further details are represented in Table 2.
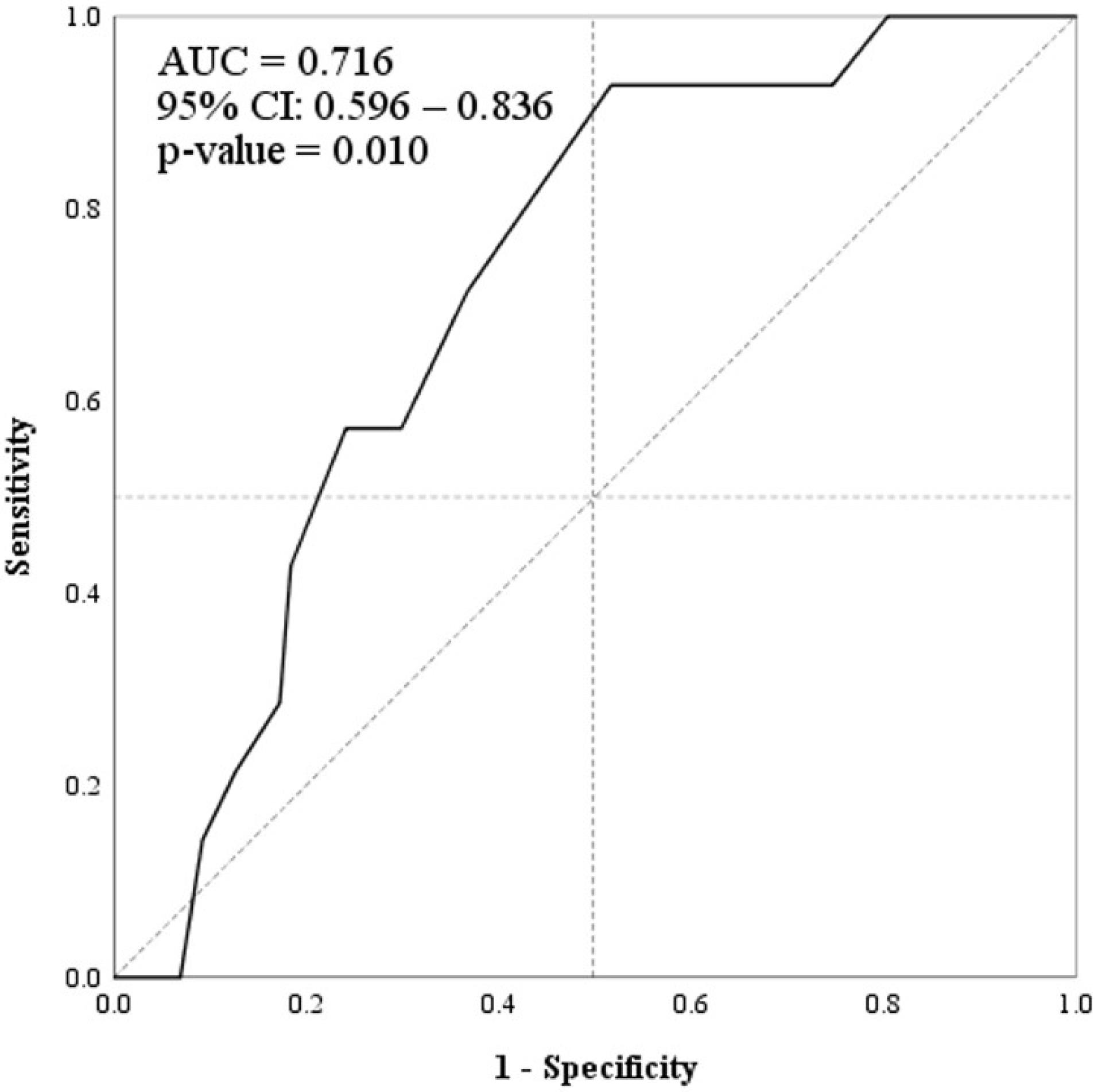
Figure 2.
Receiver Operating Characteristic Curve of “Maximum Diameter of Embolized Artery” (Solid Line) for Discriminating “12-month Hemoptysis Recurrence”. AUC: area under the curve, CI: confidence interval
.
Receiver Operating Characteristic Curve of “Maximum Diameter of Embolized Artery” (Solid Line) for Discriminating “12-month Hemoptysis Recurrence”. AUC: area under the curve, CI: confidence interval
Table 2.
Sensitivity, Specificity, and Predictive Abilities of Four Different Cut-offs for Arterial Diameter for Discriminating “12-month Hemoptysis Recurrence” in 101 Patients Treated by Bronchial Artery Embolization
|
|
Cut-off for Arterial Diameter
|
≥1.5 mm
(Most Sensitive)
|
≥2.0 mm
(Selected)
|
≥3.0 mm
(Predefined)
a
|
≥4.0 mm
(Most Specific)
|
|
+
|
-
|
+
|
-
|
+
|
-
|
+
|
-
|
| Recurrent hemoptysis |
+ |
14 |
0 |
13 |
1 |
0 |
14 |
0 |
14 |
| - |
70 |
17 |
45 |
42 |
5 |
82 |
0 |
87 |
| Sensitivity (%) |
100.0 |
92.8 |
0.0 |
0.0 |
| Specificity (%) |
19.5 |
48.2 |
94.2 |
100.0 |
| PPV (%) |
16.6 |
22.4 |
0.0 |
–* |
| NPV (%) |
100.0 |
97.6 |
85.4 |
86.1 |
| LR + |
1.24 |
1.79 |
0.0 |
–* |
| LR- |
0.0 |
0.14 |
1.06 |
1.00 |
| DOR |
–* |
12.13 |
0.0 |
–* |
DOR: diagnostic odds ratio, LR + : positive likelihood ratio, LR-: negative likelihood ratio, NPV: negative predictive value, PPV: positive predictive value.
*Unable to compute (division by zero).
aThe diameter regarded as abnormal in the literature.19
In univariate logistic regression models, fibrosis, destructive changes, and maximum arterial diameter significantly predicted the 12-month recurrence of hemoptysis. Yet, multivariate models showed that only destructive changes (OR = 5.40 [95% CI: 1.41–20.58]) and arterial diameter ≥ 2 mm (12.51 [1.51–103.59]) were independently associated with the 12-month recurrence (Table 3). We also found destructive changes (9.47 [2.04–43.91]) and arterial diameter ( + 1 mm increments, 4.42 [1.03–19.00]) as independent predictors of 9-month recurrence. Figure 3 demonstrates the Kaplan-Meier curves for recurrence-free time in the participants based on destructive changes and arterial diameter.
Table 3.
Significant Univariate and Multivariate Predictors of 12-months Hemoptysis Recurrence after Bronchial Artery Embolization
|
Predictor
|
Univariate Model
|
Multivariate Model a
|
|
OR (95% CI)
|
P
Value
|
OR (95% CI)
|
P
Value
|
| Diameter of artery ≥ 2 mm |
12.13 (1.52–96.82) |
0.019 |
12.51 (1.51–103.59) |
0.019 |
| Destructive changes |
5.18 (1.51–17.78) |
0.009 |
5.40 (1.41–20.58) |
0.013 |
| Fibrotic changes |
3.89 (1.13–13.42) |
0.031 |
|
|
CI: confidence interval, OR: odds ratio.
aMultivariate Cox logistic regression modeling with backward selection method.
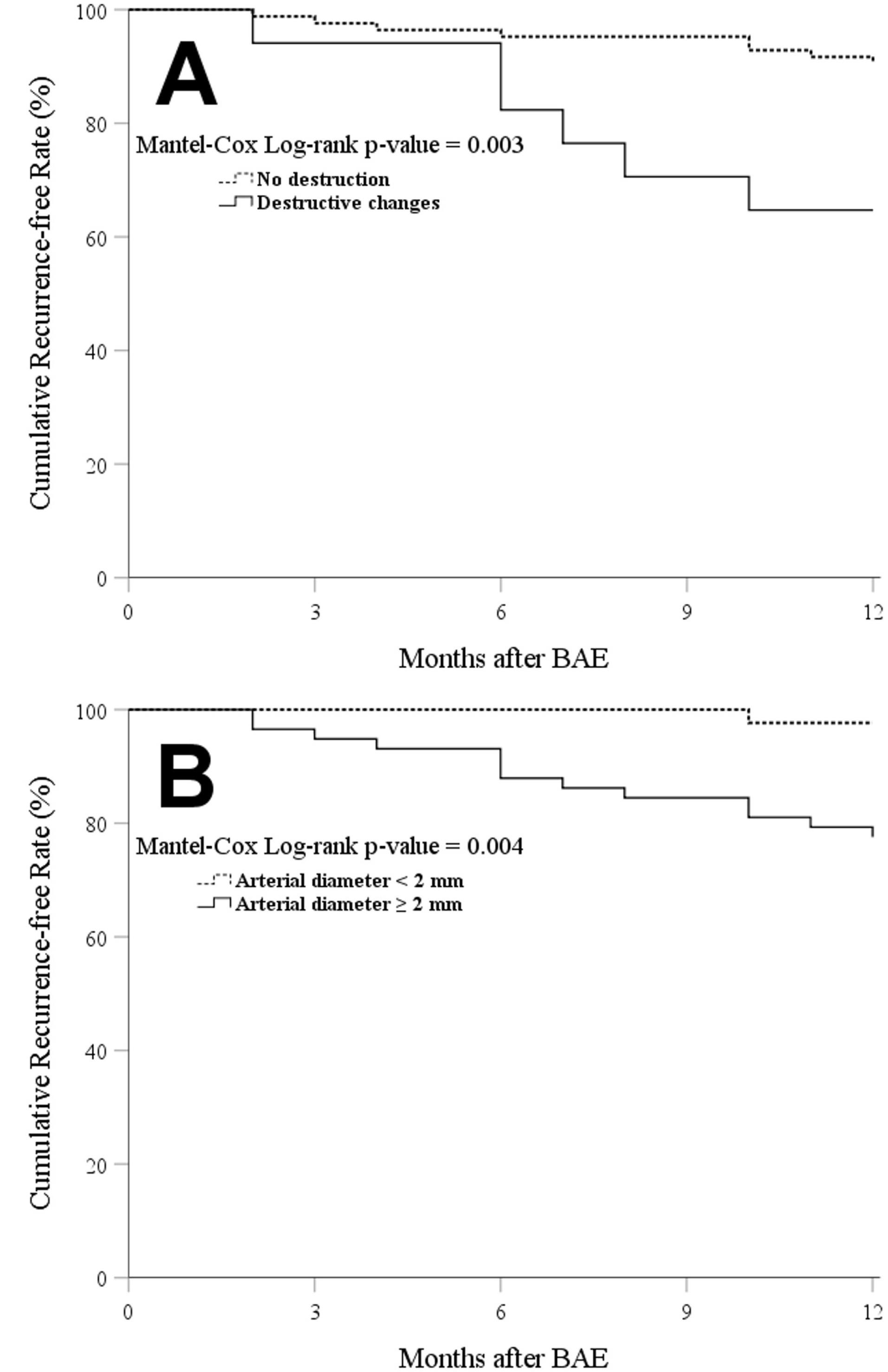
Figure 3.
Kaplan–Meier Curves for Cumulative Hemoptysis Recurrence-free Rates Based on: (A) Presence of Lung Destruction; and (B) Maximum Diameter of Embolized Artery. BAE: bronchial artery embolization
.
Kaplan–Meier Curves for Cumulative Hemoptysis Recurrence-free Rates Based on: (A) Presence of Lung Destruction; and (B) Maximum Diameter of Embolized Artery. BAE: bronchial artery embolization
Discussion
Hemoptysis has different etiologies in different regions. Consistent with previous observations in Iran and other Eastern countries, TB (30.7%) and bronchiectasis (57.4%) were the most prevalent etiologies of hemoptysis in our cohort.4,21,26,27 We studied a population in northeastern Iran, where various socioeconomic factors might constrain the affected people to neglect their hemoptysis or to delay the clinical visit, which consequently might lead to more severe morbidity, and higher rates of treatment failure or hemoptysis-related mortality.13-17
In the current study, bleeding was immediately controlled in all patients. According to the literature, the overall immediate clinical success rate of BAE is 70%–100%.1,19 With technologic advances and in the hands of an expert and skilled interventionist, high technical success rates can be achieved.19,28 The hemoptysis control rate was approximately 100% after one month and 86% after one year in our cohort.
Recurrence of hemoptysis after a successful BAE is not uncommon, ranging from 10% to 57%.1,19 Despite high immediate clinical success rate in our cohort, hemoptysis recurred in 13.9% (95% CI: 8.2–21.6) of participants during 12 months of follow-up. Previous studies in Iran reported 12-month recurrence rates of 15.5%26 and 36.7%,29 which is higher than our observation. The rate might be different due to differences in the sample population, research method, BAE technique, and embolizing material.22 The former recurrence rate was observed after BAE without microcatheterization, and the latter was observed in patients with pulmonary TB.
So far, diverse predictors of hemoptysis recurrence are reported. The etiology of hemoptysis was not associated with the recurrence rate in our study. As we observed, destructive changes in the lung and the diameter of the culprit artery were independently associated with the recurrent hemoptysis. Previous studies concluded that hemoptysis would recur due to incomplete embolization, non-bronchial systemic artery involvement, recanalization of embolized arteries, and neovascularization due to inflammation and underlying disease progression.1,4,16,20,21 Incomplete embolization and non-bronchial systemic artery involvement were risk factors for early recurrence ( < 1 month), while recanalization and neovascularization were associated with late recurrence ( > 1 month).20
The diameter of the embolized artery was independently associated with the recurrence of hemoptysis in our cohort. As shown previously, relatively large embolized arteries, compared with smaller arteries, were more susceptible to recanalization after BAE.23 Generally, a bronchial artery diameter larger than 3 mm is considered abnormal.19 We detected 2 mm as the optimal cutoff for arterial diameter for discriminating recurrent hemoptysis, and found that a diameter larger than 2 mm was the strongest predictor of 12-month recurrence.
Although TB, bronchiectasis, or cancer, as the etiologies, were not significantly associated with recurrence in our cohort, we found that the hemoptysis would probably recur if the lung parenchyma was destructed. This observation was in line with the studies by Kim et al22 and Wang et al24 which reported lung destruction as a risk factor of recurrent hemoptysis after BAE in patients with pulmonary TB. According to Wang et al, post-TB bronchiectasis patients with destructive changes were at quadruple higher risk of recurrent hemoptysis than patients without destroyed lung.24 Lu et al also found lung destruction as a predictor of early recurrence of hemoptysis.20 On the contrary, TB-destroyed lung was not associated with recurrent hemoptysis ina study byHwang et al30 This discrepancy might emanate from differences in BAE technique, TB prevalence, study timeframe, and demographics of studied populations.22 TB-destroyed lung was found to be a poor prognostic factor of hemoptysis that is associated with decreased lung function and increased mortality.22,24
Apart from methodological limits, such as information bias, a draw-back of the present study is lack of data pertaining to the volume and severity of hemoptysis, and potential confounding factors, such as socioeconomic status, comorbidities (e.g. diabetes mellitus, and chronic liver diseases), and medical treatment for etiologic diseases (before and after BAE). We did not assess inflammatory markers, since they are highly variable in long-term.21 We followed recommended standards for BAE (device calibration, use of PVA, super-selective embolization, and post-BAE angiography) to reduce technical bias.1 To minimize the information bias, two radiologists independently assessed the images, and the quantitative data (e.g. arterial diameter) were averaged. There were no disparities in assessment of qualitative features (e.g. presence of destructive changes) by the two radiologists (not reported). The radiologists, unaware of the recurrence status, were not blinded to the etiologic disease, or technical and clinical outcomes of BAE.
Conclusion
During the first year after BAE, hemoptysis recurred in 13.9% of the participants in this cohort. According to our observation, patients should be followed up at least one year after BAE, particularly cases with destroyed lungs and embolized arteries wider than 2.0 mm. Further studies with larger sample sizes might corroborate our observation, and are needed to investigate factors affecting long-term recurrence of hemoptysis.
Acknowledgements
This manuscript is based on medical dissertation of the first author (SS) in Radiology. The study received no public or private funds. The authors would like to thank the staff of Ghaem and Imam Reza hospitals, Mashhad, Iran.
Competing Interests
The authors declare that they have no conflict of interest.
Ethical Approval
Following the Helsinki Declaration and the STROBE guideline, we conducted this study with approval of Research Ethics Committee of Mashhad University of Medical Sciences (reference number: IR.MUMS.IRH.REC.1401.041). All participants freely gave informed consent prior to participation.
References
- Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol 2017; 23(4):307-17. doi: 10.5152/dir.2017.16454 [Crossref] [ Google Scholar]
- Dabó H, Gomes R, Marinho A, Madureira M, Paquete J, Morgado P. Bronchial artery embolisation in management of hemoptysis--a retrospective analysis in a tertiary university hospital. Rev Port Pneumol (2006) 2016; 22(1):34-8. doi: 10.1016/j.rppnen.2015.09.001 [Crossref] [ Google Scholar]
- Chun JY, Morgan R, Belli AM. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol 2010; 33(2):240-50. doi: 10.1007/s00270-009-9788-z [Crossref] [ Google Scholar]
- Li H, Ding X, Zhai S, Gao K. A retrospective study on the management of massive hemoptysis by bronchial artery embolization: risk factors associated with recurrence of hemoptysis. BMC Pulm Med 2023; 23(1):87. doi: 10.1186/s12890-023-02371-1 [Crossref] [ Google Scholar]
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002; 22(6):1395-409. doi: 10.1148/rg.226015180 [Crossref] [ Google Scholar]
- Shin BS, Jeon GS, Lee SA, Park MH. Bronchial artery embolisation for the management of haemoptysis in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis 2011; 15(8):1093-8. doi: 10.5588/ijtld.10.0659 [Crossref] [ Google Scholar]
- Chalmers JD, Polverino E, Crichton ML, Ringshausen FC, De Soyza A, Vendrell M. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis Registry (EMBARC). Lancet Respir Med 2023; 11(7):637-49. doi: 10.1016/s2213-2600(23)00093-0 [Crossref] [ Google Scholar]
- Nigro M, Laska IF, Traversi L, Simonetta E, Polverino E. Epidemiology of bronchiectasis. Eur Respir Rev 2024; 33(174):240091. doi: 10.1183/16000617.0091-2024 [Crossref] [ Google Scholar]
- Bagheri R, Haghi SZ, Fattahi Masoum SH, Bahadorzadeh L. Surgical management of bronchiectasis: analysis of 277 patients. Thorac Cardiovasc Surg 2010; 58(5):291-4. doi: 10.1055/s-0030-1249941 [Crossref] [ Google Scholar]
- Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J 2019; 54(3):1900655. doi: 10.1183/13993003.00655-2019 [Crossref] [ Google Scholar]
- Doosti A, Nasehi M, Moradi G, Roshani D, Sharafi S, Ghaderi E. The pattern of tuberculosis in Iran: a national cross-sectional study. Iran J Public Health 2023; 52(1):193-200. doi: 10.18502/ijph.v52i1.11682 [Crossref] [ Google Scholar]
- Fallahzadeh H, Khazaei Z, Lari Najafi M, Rahimi Pordanjani S, Goodarzi E. Distribution incidence, mortality of tuberculosis and human development index in Iran: estimates from the global burden of disease study 2019. BMC Public Health 2023; 23(1):2404. doi: 10.1186/s12889-023-17114-4 [Crossref] [ Google Scholar]
- Crane M, Scott N, O’Hara BJ, Aranda S, Lafontaine M, Stacey I. Knowledge of the signs and symptoms and risk factors of lung cancer in Australia: mixed methods study. BMC Public Health 2016; 16:508. doi: 10.1186/s12889-016-3051-8 [Crossref] [ Google Scholar]
- Said K, Hella J, Mhalu G, Chiryankubi M, Masika E, Maroa T. Diagnostic delay and associated factors among patients with pulmonary tuberculosis in Dar es Salaam, Tanzania. Infect Dis Poverty 2017; 6(1):64. doi: 10.1186/s40249-017-0276-4 [Crossref] [ Google Scholar]
- Smith SM, Campbell NC, MacLeod U, Lee AJ, Raja A, Wyke S. Factors contributing to the time taken to consult with symptoms of lung cancer: a cross-sectional study. Thorax 2009; 64(6):523-31. doi: 10.1136/thx.2008.096560 [Crossref] [ Google Scholar]
- Burke CT, Mauro MA. Bronchial artery embolization. Semin Intervent Radiol 2004; 21(1):43-8. doi: 10.1055/s-2004-831404 [Crossref] [ Google Scholar]
- Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med 2000; 28(5):1642-7. doi: 10.1097/00003246-200005000-00066 [Crossref] [ Google Scholar]
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010; 80(1):38-58. doi: 10.1159/000274492 [Crossref] [ Google Scholar]
- Sopko DR, Smith TP. Bronchial artery embolization for hemoptysis. Semin Intervent Radiol 2011; 28(1):48-62. doi: 10.1055/s-0031-1273940 [Crossref] [ Google Scholar]
- Lu GD, Zu QQ, Zhang JX, Zhou CG, Xia JG, Ye W. Risk factors contributing to early and late recurrence of haemoptysis after bronchial artery embolisation. Int J Tuberc Lung Dis 2018; 22(2):230-5. doi: 10.5588/ijtld.17.0543 [Crossref] [ Google Scholar]
- Yan HT, Lu GD, Huang XZ, Zhang DZ, Ge KY, Zhang JX. Development of a model to predict recurrence after bronchial artery embolization for non-cancer related hemoptysis. BMC Pulm Med 2021; 21(1):419. doi: 10.1186/s12890-021-01790-2 [Crossref] [ Google Scholar]
- Kim SW, Lee SJ, Ryu YJ, Lee JH, Chang JH, Shim SS. Prognosis and predictors of rebleeding after bronchial artery embolization in patients with active or inactive pulmonary tuberculosis. Lung 2015; 193(4):575-81. doi: 10.1007/s00408-015-9728-4 [Crossref] [ Google Scholar]
- Ryuge M, Hara M, Hiroe T, Omachi N, Minomo S, Kitaguchi K. Mechanisms of recurrent haemoptysis after super-selective bronchial artery coil embolisation: a single-centre retrospective observational study. Eur Radiol 2019; 29(2):707-15. doi: 10.1007/s00330-018-5637-2 [Crossref] [ Google Scholar]
- Wang LL, Lu HW, Li LL, Jiang S, Xu JF. Destroyed lung contributes to the recurrence of hemoptysis after bronchial artery embolization in patients with post-tuberculosis bronchiectasis. J Infect Public Health 2024; 17(7):102446. doi: 10.1016/j.jiph.2024.05.003 [Crossref] [ Google Scholar]
- Angle JF, Siddiqi NH, Wallace MJ, Kundu S, Stokes L, Wojak JC. Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 2010; 21(10):1479-86. doi: 10.1016/j.jvir.2010.06.014 [Crossref] [ Google Scholar]
- Keshmiri MS, Shafaghi S, Sharif-Kashani B, Sadoughi A, Ghorbani F, Naghashzadeh F. Preemptive non-selective bronchial artery angioembolization to reduce recurrence rate of hemoptysis. Multidiscip Respir Med 2020; 15(1):723. doi: 10.4081/mrm.2020.723 [Crossref] [ Google Scholar]
- Seyyedi SR, Sadeghipour P, Sadr M, Shafe O, Moosavi J, Aloosh O. Outcomes and complications of bronchial angioembolization in patients with massive hemoptysis. Tanaffos 2019; 18(4):310-4. [ Google Scholar]
- Meléndez-Torres JR, Padua-y-Gabriel A, Velasco-Rodriguez VM, Martinez-Ordaz V, Sánchez-Cabral O, Cicero-Sabido R. Survival after bronchial artery embolization in massive hemoptysis: experience in 24 cases. J Bronchology Interv Pulmonol 2003; 10(1):17-21. doi: 10.1097/00128594-200301000-00004 [Crossref] [ Google Scholar]
- Seyyedi SR, Tabarsi P, Sadr M, Aloosh O, Keshmiri MS, Abedini A. Bronchial angioembolization for management of hemoptysis due to pulmonary tuberculosis. Tanaffos 2021; 20(2):134-9. [ Google Scholar]
- Hwang HG, Lee HS, Choi JS, Seo KH, Kim YH, Na JO. Risk factors influencing rebleeding after bronchial artery embolization on the management of hemoptysis associated with pulmonary tuberculosis. Tuberc Respir Dis (Seoul) 2013; 74(3):111-9. doi: 10.4046/trd.2013.74.3.111 [Crossref] [ Google Scholar]