Arch Iran Med. 28(5):257-263.
doi: 10.34172/aim.33355
Original Article
Efficacy Of N-Acetyl-Cysteine as Adjuvant Therapy for Diabetic Foot Osteomyelitis: An Open-Label Randomized Controlled Trial
Laya Hooshmand Gharabagh Data curation, Formal analysis, Investigation, Project administration, Validation, Visualization, Writing – original draft, 1 
Mehdi Heydaroghli Data curation, Formal analysis, Investigation, Project administration, Validation, Visualization, Writing – review & editing, 2 
Ayda Esmaeili Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 3, 4, * 
Author information:
1Department of Internal Medicine, School of Medicine, Urmia University of Medical Sciences, Imam Khomeini Hospital, Urmia, Iran
2Student Research Committee, Urmia University of Medical Sciences, Urmia, Iran
3Department of Clinical Pharmacy, School of Pharmacy, Urmia University of Medical Sciences, Urmia, Iran
4Patient Safety Research Center, Clinical Research Institute, Urmia University of Medical Sciences, Urmia, Iran
Abstract
Background:
Biofilm formation by bacteria on the lower limb arises from reduced peripheral arterial blood flow, which can lead to the failure of antibiotic therapy or require longer duration of intravenous antibiotic therapy in diabetic foot infection-associated osteomyelitis. N-acetyl cysteine (NAC), an agent known to prevent and treat biofilm-related infections, was used as a novel strategies beside antibiotic therapy in osteomyelitis of diabetic foot with the aim of accelerating the response to antibiotic therapy regimen.
Methods:
To assess the synergistic effect of NAC with antibiotic therapy, patients with diabetic foot osteomyelitis (DFO) (grade III or IV Wagner) were randomly assigned to either NAC 600 mg effervescent tablet twice daily for 2 weeks or the control group. Clinical and laboratory data, including white blood cell with differentiation and inflammatory factors (ESR and CRP) were measured at baseline (time 0), after one week and after three weeks of initiating the intervention.
Results:
Fifty-three eligible patients completed the study. All evaluated infectious-related laboratory parameters showed significant reductions in the NAC group compared to control (P<0.05), except for lymphocyte proportion and NLR (P; 0.11 and 0.84, respectively). The drop rate of ESR and CRP were accelerated by NAC compared to the control group (-49.44±6.04 vs -7.17±3.99; -44.43±4.21 vs -14.02±4.05, respectively, P<0.05).
Conclusion:
In order to accelerate antibiotic responses and the trend of reduction in infectious inflammatory markers during the therapy, oral NAC 600 mg twice daily may be considered in the treatment protocol of patients with DFO.
Keywords: Inflammatory markers, Diabetic foot osteomyelitis, N-acetyl cysteine, Optimizing treatment response
Copyright and License Information
© 2025 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Hooshmand Gharabagh L, Heydaroghli M, Esmaeili A. Efficacy of N-acetyl-cysteine as adjuvant therapy for diabetic foot osteomyelitis: an open-label randomized controlled trial. Arch Iran Med. 2025;28(5):257-263. doi: 10.34172/aim.33355
Introduction
Diabetic foot ulcer (DFU) is a devastating complication in diabetic patients with 4%‒10% prevalence, especially in poor glycemic control and geriatric patients. If bone infection is present in DFU (osteomyelitis), it is classified as a Wagner’s grade 3 and above.1 Approximately three fifths of DFU are infected.2 Osteomyelitis is reported in about 50% and 10‒15% of severe and moderate infected DFUs, respectively.3 Diabetic foot osteomyelitis (DFO) is categorized as non-device-related bacterial biofilm infection. The failure of and/or need for prolonged duration of intensive doses of intravenous antibiotic therapy are common in DFO. Impairment of peripheral arteries, especially in the lower limbs and high probability of forming biofilm on lower limb bones have led to continued investigation of new adjuvants to improve response to antibiotic therapy.
N- acetyl-cysteine (NAC) is a known thiol compound and N-acetylated endogenous amine acid L-cysteine, has a wide range of uses, including as mucolytic agent, and an antidote in acetaminophen toxicity.4 The multiple mechanisms of action have been described in literature, including (1) antioxidant function through balancing the redox, (2) neutralizing reactive oxygen species (ROS), (3) reduction of inflammatory cytokine such as IL6, IL1β, and TNF-α, and (4) vasodilation through inducing nitric oxide (NO).5,6 Additionally, NAC has shown bactericidal properties against Staphylococcus aureus, Enterococcus faecalis, and Corynebacterium ammoniagenes in studies and prevented biofilm formation of Gram-positive and Gram-negative bacteria in in-vivo studies.7-10 Besides, NAC shows beneficial effects in dermatology conditions such as wound healing.11 Intraperitoneal administration of NAC in diabetic and non-diabetic mice for 5 days increased angiogenesis through vascular endothelial growth factor (VEGF) and resulted in faster wound healing.12 However, new studies designed for human populations are needed to confirm its efficacy for this specific purpose.
Considering the above-mentioned pleiotropic actions of NAC, it is hypothesized that NAC can improve therapy response in diabetic foot infection, accelerating wound healing, inhibiting microbial biofilm formation and shortening the course of antibiotic therapy.
According to recent clinical studies, serial (at least weekly) measurements of C-reactive protein (CRP), white blood cell count (WBC), neutrophil to lymphocyte ratio (NLR) and erythrocyte sedimentation rate (ESR) during treatment guide us to assess response to intravenous (IV) antibiotic therapy in osteomyelitis and are additionally helpful to determine when to switch over from intravenous to oral therapy.13-16
Therefore, the current study was designed to evaluate the effects of oral NAC on response to antibiotic therapy based on the inflammatory factors including ESR and CRP in patients with grade III and IV DFU according to Wagner’s classification.1
Materials and Methods
Study Design
This randomized, single-center, open-label clinical trial enrolled all patients with DFO admitted to the endocrinology ward of a major academic hospital affiliated to Urmia Medical Sciences University (UMSU) in Urmia, Iran, between December 1, 2020, and November 30, 2022.
Study Participants
Inclusion Criteria
Patients aged 18 years or older with DFO, classified as grade III or IV Wagner, were enrolled in the current study. For all patients, bone involvement was confirmed by magnetic resonance imaging (MRI). Vascular evaluation of the lower limbs was done with color Doppler ultrasonography to rule out any thrombosis or any severe vascular involvement. All patients were evaluated by one plastic surgeon within 1‒2 days of admission to assess any need for surgical debridement or amputation (partial, digital or total). The surgeon decided on debridement and/or amputation based on the intensity of devitalized and necrosis of tissue. Clinical signs including pain, erythema, and edema as signs of inflammation, increase or no changes in purulent exudate, foul odor, and deterioration in the appearance of the wound were assessed by the physician to determine the intensity of antibiotic therapy.
Participants with a PEDIS score (Perfusion, Extent, Depth, Infection and Sensation) ≥ 7 were eligible to enter the study, which indicates that the patient is high risk and should have intensive antibacterial regimen intervention.18
Exclusion criteria
(1) Already being treated with any known supplements that have anti-inflammatory and anti-oxidative effects such as Vitamin C, D, E and A, herbal supplements such as curcumin, etc.; (2) administration of NAC at least 2 weeks before hospitalization; (3) history of hypersensitivity reaction to NAC or sulfur products; (4) Other causes of ulcers besides diabetes including trauma, skin disease, and rheumatologic disease; (5) patients with active malignancy or who received chemotherapy or radiotherapy within the previous year; (6) patients with a Glasgow Coma Scale score of 12 or less and those who could not tolerate oral agents; (7) pregnancy or breastfeeding; (8) patients on immunosuppressant medications such as corticosteroids ( ≥ 40 mg prednisolone equivalent dose), mycophenolate, cyclosporine, tacrolimus, or rituximab, which interfere with wound healing; (9) hospitalization for a DFU in the past month and receiving injection antibiotic treatment; (10) patients with chronic kidney disease with creatinine clearance < 15 mL/min19 or undergoing dialysis, and those with decompensated liver disease (considered to be immunocompromised20); (11) substance addiction; (12) those requiring foot amputation or needing revascularization or amputation (partial, digital or total), and those with DFU in grade V Wagner.21
Sample Size and Randomization
Based on the mean wound healing score after surgery in a previous study by Oguz et al (31.89 ± 2.26 in the control group and 33.98 ± 2.14 in the N-acetylcysteine group), considering a 95% confidence interval (Z1-α/2 = 1.96) and a power of 90% (Z1-β = 1.28), we calculated a sample size of 28 patients in each group using the following formula22:
Eligible participants were randomized using block randomization; given the total sample size of 56 patients, 14 blocks of 4 were used. The randomized list of numbers 1 to 56 was randomly divided into two groups of A (intervention group) and B (control group), and the subjects were alternately allocated into group A or B, yielding 28 people in each group. The intervention group received NAC in oral effervescent form 600 mg twice daily for 14 days, in concurrent use with intravenous antibiotic medications vancomycin or teicoplanin (if allergic to vancomycin), ciprofloxacin and carbapenem (imipenem- cilastatin or meropenem depending on availability). This dosage of NAC was chosen from trials related to pulmonary infections.23-25
According to the DFO treatment guideline, the length of systemic antibiotic therapy was considered to be 3 weeks. The control group only received rational intravenous antibiotic medications. Bacterial culture from the wound and blood were obtained on the day of admission for all patients. The results of culture were used to identify the pathogenic bacteria in wound discharge, and guide appropriate and effective antibiotics. Patients’ demographic data including age, gender, height and weight for calculating body mass index (BMI), number of wounds, onset and duration of foot ulcer, duration of diabetes, and any other medical history were gathered. Laboratory data such as blood glucose, blood urine nitrogen (BUN), serum creatinine, and complete blood cell count and differential were assessed at the beginning and after three weeks. In addition, baseline CRP and ESR levels were measured and rechecked after one week and around the time of discharge (19‒23 days of admission, three weeks). All patients were evaluated by the investigators daily and any possible side effects of NAC were recorded using Naranjo as an Adverse Drug Reaction Probability Scale.26 At least 80% adherence to medication was considered as acceptable compliance.27
Statistical Analysis
All the statistical analyses were conducted using the SPSS statistics software (version 20.0, SPSS Inc., Chicago, IL, USA). Categorical data was expressed by frequency (percentage). Normally and not normally distributed continuous values were presented as mean ± SD and/or median (interquartile range), respectively. The normality assumption of data was assessed with the Kolmogorov–Smirnov test and Q-Q plot. Then, the means of variables with normal or non-normal distribution were compared between the two groups using parametric or nonparametric tests including independent t test/ ANCOVA or Mann-Whitney U test, respectively. For comparing the within-group changes, we used a paired sample t test or Wilcoxon Rank test for variables with normal or non-normal distribution, respectively. The per-protocol method was used for data analysis. A P value < 0.05 was considered as significant.
Results
Patient Characteristics
A total of 56 patients with confirmed DFO were enrolled in current study; 28 patients were assigned to each group. Three patients in the NAC group were excluded and the other participants in both groups completed the trial (Figure 1). The patients’ demographic and diagnostic information of DFO is listed in Table 1. The patients who completed the study improved clinically over the hospitalization course and were discharged with acceptable clinical status on oral antibiotics agents. There was no statistically significant difference between the groups regarding their characteristics except for age (NAC group: 68.00 ± 13.78, control group: 57.74 ± 8.32; P value = 0.003).
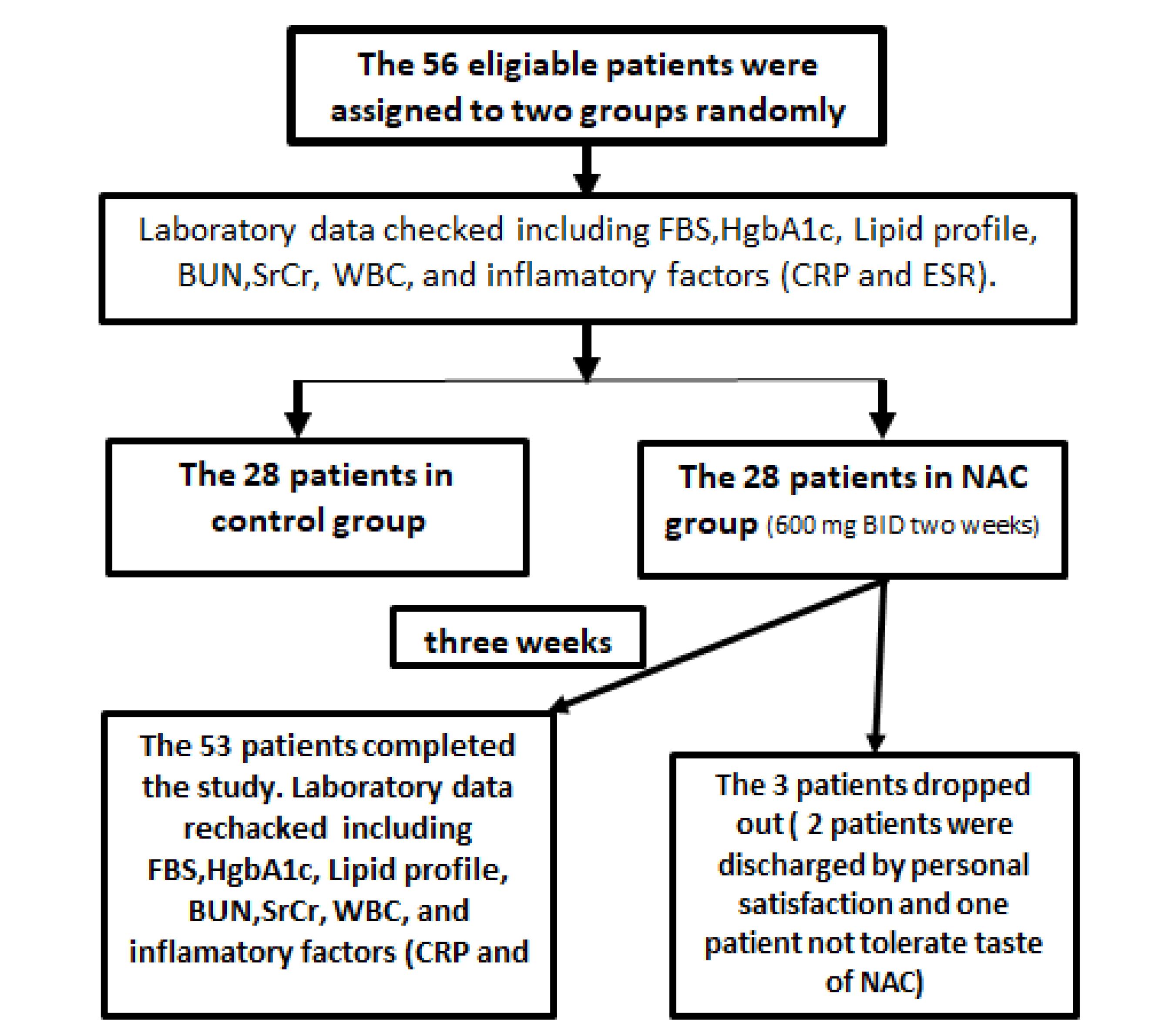
Figure 1.
Flow Diagram of Study
.
Flow Diagram of Study
Table 1.
Patients; Characteristics at Baseline
|
Group (n=60)
|
NAC Group (n=25)
|
Control Group (n=28)
|
P
Value*
|
| Age, year, mean ± SD |
68.00 ± 13.78 |
57.74 ± 8.32 |
0.003 |
| Sex (F/M) |
17/8 |
16/12 |
0.42 |
| BMI (kg/m2) |
26.37 ± 2.38 |
27.88 ± 2.75 |
0.06 |
| Wagner classification grade of DFO (grade 3/grade 4) |
11/14 |
18/10 |
0.55 |
| Ulcer duration (months) |
1.75 ± 0.96 |
2.21 ± 0.63 |
0.12 |
| Number of DFU |
|
|
|
| 1 |
11 |
18 |
0.55 |
| > 1 |
14 |
10 |
| Regimen of antibiotic therapy |
|
|
|
| Vancomycin + meropenem |
10 |
15 |
|
| Vancomycin + ciprofloxacin + meropenem |
11 |
9 |
|
| Others |
4 |
3 |
|
| Microbiologic results of wound swab culture |
|
|
|
| Negative |
12 |
8 |
|
| Positive |
13 |
20 |
|
DFO, Diabetic foot osteomyelitis; BMI, Body Mass Index; DFU, Diabetic foot ulcer; n, Number of patients.
* P value < 0.05 is significant.
According to our findings, contrary to the control group, WBC and the N/L ratio significantly improved over three weeks’ therapy compared to baseline value in the NAC group (WBC at the end of study in NAC group: 7807.80 ± 2720.48 vs. control group: 8530 ± 2775.89; Neutrophil ratio in NAC group: 63.78% ± 8.38% vs. control group: 74.32% ± 8.77%; P< 0.01). However, there was no statistically significant difference in the N/L ratio changes between the two groups (N/L ratio change in NAC group: 7.25 ± 3.99 to 5.12 ± 2.23 vs. control group: 10.34 ± 9.17 to 8.5 ± 5.19; P value = 0.84; Table2).
Table 2.
Comparing Laboratory Data at Baseline and End of Study in NAC and Control Groups
|
|
Patients NAC 600 mg BID for 2 weeks (n=25)
|
Control Group (n=28)
|
P
Value Between Groups at Baseline
|
P
Value of Changes of Variables Between Groups at End
|
|
Baseline
|
End
|
P
Value
|
Baseline
|
End
|
P
Value
|
| Hgb-A1C (%) |
9.53 ± 1.91 |
9.13 ± 1.99 |
0.001 |
9.44 ± 1.04 |
9.44 ± 1.04 |
0.63 |
0.33 |
0.001* |
| FBS (mg/dL) # |
219.48 ± 84.67 |
152.36 ± 29.75 |
< 0.001 |
179.85 ± 38.8 |
162.61 ± 28.93 |
0.006 |
0.04 |
0.004¥ |
| WBC (per microliter) |
10223.36 ± 3039.70 |
7807.80 ± 2720.48 |
0.001 |
9002.50 ± 2289.6 |
8530 ± 2775.89 |
0.22 |
0.10 |
0.01* |
| Neutrophil % |
75.72 ± 8.38 |
63.78 ± 8.38 |
< 0.001 |
78.32 ± 8.60 |
74.32 ± 8.77 |
0.001 |
0.27 |
< 0.001* |
| Lymphocyte % |
12.96 ± 5.46 |
14.96 ± 6.87 |
0.02 |
11.18 ± 6.08 |
14.66 ± 6.40 |
0.32 |
0.26 |
0.11* |
| N/L ratio |
7.25 ± 3.99 |
5.12 ± 2.23 |
0.003 |
10.34 ± 9.17 |
8.5 ± 5.19 |
0.14 |
0.13 |
0.84* |
Hgb-A1C, Hemoglobin A1C; FBS, Fast blood sugar; N, Neutrophil; NLR, Neutrophil / Lymphocyte ratio; n, Number of patients.
* Comparison made with independent T-test.
¥ Comparison made with ANCOVA test.
# Comparison made with Mann-Whitney U test.
P value < 0.05 is significant.
Table 3 compares the assessed inflammatory variables including ESR and CRP at baseline, after one week and at discharge time (one week after the end of intervention) in each group and within groups. According to analysis, both ESR and CRP in patients on NAC decreased significantly after one week and at discharge time (ESR at baseline: 87.08 ± 28.90 vs. after a week: 76.48 ± 26.24 vs. at discharge: 37.64 ± 22.36; P value < 0.001) (CRP at baseline: 73.57 ± 26.53 vs. after a week: 61.14 ± 25.22 vs. at discharge: 29.13 ± 21.05; P < 0.001), while in the control group, non-significant changes were observed in ESR and CRP after one week of starting only systemic antibiotics (P = 0.09 and 0.05, respectively) and the rate of decline in ESR and CRP level over the length of admission were slower (Figure 2).
Table 3.
Comparing Inflammatory Markers at Baseline and Hospital Discharge in NAC and Control Groups
|
|
NAC Group
|
Mean of Changes
|
P
Value*
|
Control Group
|
Mean of Changes
|
P
Value*
|
P
Value¥ Between Groups
|
|
Mean±SD
|
Mean±SE
|
Mean±SD
|
Mean±SE
|
| ESR |
Baseline |
87.08 ± 28.90 |
|
‒ |
54.07 ± 19.51 |
|
|
< 0.001 |
| After one week of starting intervention |
76.48 ± 26.24 |
-10.59 ± 2.41 |
< 0.001 |
52.53 ± 18.24 |
-1.53 ± 0.87 |
0.09 |
0.001 |
| Hospital discharge (after 3 weeks) |
37.64 ± 22.36 |
-49.44 ± 6.04 |
< 0.001 |
46.89 ± 21.87 |
-7.17 ± 3.99 |
0.08 |
| CRP |
Baseline |
73.57 ± 26.53 |
|
|
63.97 ± 27.73 |
|
|
0.18 |
| After one week of starting intervention |
61.14 ± 25.22 |
-12.43 ± 2.41 |
< 0.001 |
61.14 ± 25.22 |
-2.11 ± 0.51 |
0.05 |
< 0.001 |
| Hospital discharge (after 3 weeks) |
29.13 ± 21.05 |
-44.43 ± 4.21 |
< 0.001 |
49.94 ± 30.59 |
-14.02 ± 4.05 |
0.002 |
NAC, n-acetyl cysteine; ESR, Erythrocyte sedimentation rate; CRP, C-reactive protein; n, number of patients.
* P value of comparison within group.
¥ P value of comparison between groups.
P value < 0.05 is significant.
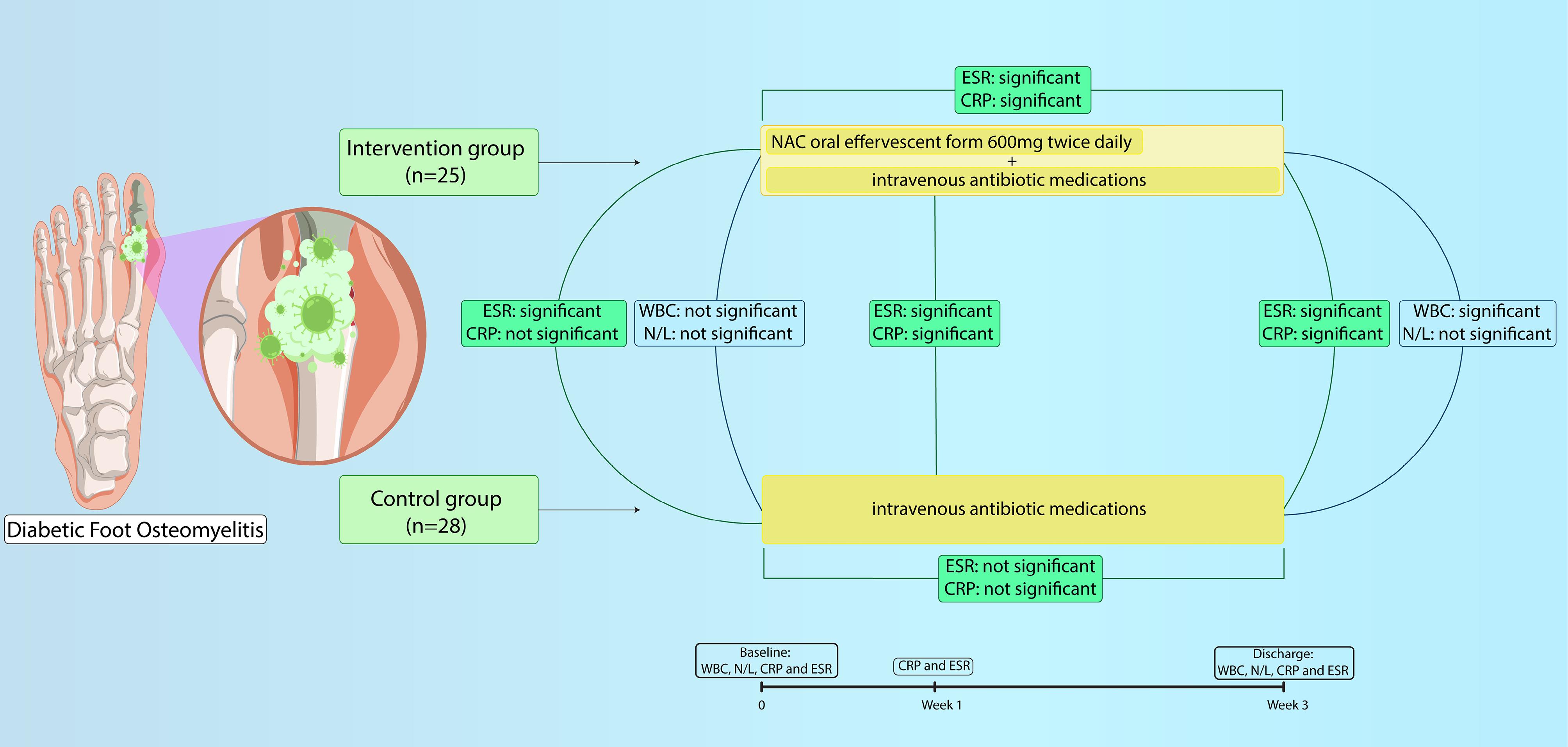
Figure 2.
Study process and results illustration
.
Study process and results illustration
One patient stopped receiving therapy due to the unpleasant taste of effervescent NAC. Also, two patients in the NAC group dropped out of study due to early discharge (personal consent) and not completing the duration of intervention.
Discussion
As an antioxidant agent with pleiotropic effects including antibacterial property simultaneously with inhibiting biofilm formation, NAC is used as an adjuvant therapy to accelerate cure in various infections such as pneumonia.23,28 To the best of our knowledge, there is no published clinical study in which NAC was used as an adjuvant therapy in DFO. Therefore, the current study was designed to evaluate the effect of NAC on the outcome of DFO antibiotic therapy. According to our findings, NAC substantially accelerated the rate of decline in ESR, CRP, WBC count, and N/L ratio which are used to evaluate response to antibiotic therapy.
The normal ranges of WBC count and NLR in the healthy population are 4500 to 11 000/m3 and 0.78 to 3.5, respectively.29 Neutrophils play a major role in regulating innate immunity, through activating other immune cells and secreting pro inflammatory cytokines which stimulate the functions of other immune cells such as dendritic cell, T and B cell lymphocytes in infections and inflammatory diseases.30 The higher NLR value and neutrophil-dominant WBC counts are proven to have independent direct correlation with morbidity and mortality rate in various diseases including infections.31 Leukocytosis and increase in NLR are valuable biomarkers for early diagnosis of infectious diseases including DFO as well as predictors for needing long-term intravenous (IV) antibiotic and hospitalization.32,33 Altay et al showed that the decrease in NLR during the first 14 days of starting treatment had a direct correlation with improvement in DFU and appropriate response to therapy IV antibiotic therapy.34 Therefore, they are reliable indicators for monitoring response to antibiotic therapy and healing of the foot ulcer.
Our study showed that patients on NAC experienced a significant reduction in WBC count and neutrophil percentage compared to the control group who received only antibiotic therapy, while there was no statistically difference in NLR value changes. Nevertheless, the decrease in NLR over two weeks in the intervention group was significant, while in the control group, it was not.
Serum inflammatory markers, including CRP, ESR, and WBC are known as diagnostic markers for diabetic foot bone infection with acceptable sensitivity and specificity (above 0.70).35 Optimal cut-off points for ESR and CRP levels as diagnostic markers have not been defined so far.36,37 However, according to Lavery et al, an ESR level more than 60 mm/h and a CRP level greater than 7.9 mg/dL (with statistically acceptable specificity and sensitivity) are optimal cut-off points for predicting osteomyelitis and the physician should consider osteomyelitis treatment for these patients.13 It is worth noting that, the CRP, WBC count, and NLR simultaneously (faster than ESR) start to decline during the therapy and rapidly help us to assess the treatment response.38 For instance, an original article by van Asten et al on 122 patients with DFO showed a direct correlation between reduction in ESR and CRP levels during therapy and acceptable clinical outcomes.38 Van Asten et al conducted another study to evaluate the value of inflammatory markers in monitoring treatment response in 35 patients hospitalized with FDO; they reported that CRP, ESR, procalcitonin, and interleukin-6 are valuable markers for assessing response to antibiotic therapy.39
Considering the value of CRP and ESR levels in treatment monitoring, patients who received NAC for 14 days had significantly lower levels of both serum inflammatory markers, CRP and ESR, on discharge than the control group. Therefore, it is concluded that NAC has a synergistic effect in combination with antibiotic therapy. The decrease rate of CRP and ESR in patients on NAC was approximately three and seven times faster in comparison with the control group. Additionally, after a week, CRP and ESR changes in the control group were not significant, meanwhile for patients on NAC, a dramatic reduction was seen even after a week. According to the results of Durak et al, CRP is the superior marker over ESR for evaluating early therapeutic response of antibiotics.40
Hence, it can be concluded that NAC might be a potent adjuvant agent beside antibiotics to accelerate response and shorten the duration of intravenous therapy; it is associated with early hospital discharge and improved patients’ compliance for the approximate 3 months of antibiotic therapy.
Limitation
This study had some limitations as follows: (1) The sample size in the current study was small which may have affected the outcome. With a larger sample size, the results can be different and the power of study is increased. (2) It is better to follow up the patients for a month and until the end of the total duration of antibiotic therapy (IV and oral; three months), and for a year to evaluate the rate of recurrence of DFO and need for readmission, which may result in decreasing the financial burden on patients with DFO, who need a longer length of stay in hospitals. (3) All patients clinically improved over 3 weeks receiving antibiotic therapy but we did not report the time to recovery of clinical features; it is recommended to evaluate the recovery time of these clinical signs, as well.
Recommendation
Based on our results, NAC is a promising candidate for accelerating inflammatory factors’ response to antibiotic therapy which may result in shortening the long antibiotic therapy; therefore, it is recommended to conduct further studies on NAC as an adjuvant therapy with antibiotics with various aims of reducing the duration of IV antibiotic therapy, length of hospital stay and recurrence of DFO over one year.
Conclusion
Oral NAC 600 mg BID along with antibiotic therapy significantly reduced the inflammatory markers of therapeutic response, including CRP and ESR. Based on this evidence, NAC may be a proper option to use as an adjutant agent in the treatment protocol of DFO with the aim of early switching from IV to oral therapy and shortening the length of hospitalization.
Acknowledgements
The authors wish to express their sincere gratitude to Clinical Researches Development Unit of Imam Khomeini Hospital, Urmia University of Medical Sciences, for English editing and Urmia University of Medical Sciences, Urmia, Iran, that made this work possible.
Competing Interests
There is no conflict of interest
Ethical Approval
This study was conducted in accordance with The Helsinki declaration.17 It was approved by the Ethics Committee of UMSU (no. IR.UMSU.HIMAM.REC.1401.047) and registered in the Iranian Registry of Clinical Trials (Identifier: IRCT20220928056051N2; https://irct.behdasht.gov.ir/trial/67875). A written informed consent form was signed by all participants in the study before entry.
Funding
This study was funded by Urmia University of Medical Sciences, Urmia, Iran., Grant agreement NO. 11220
References
- Shah P, Inturi R, Anne D, Jadhav D, Viswambharan V, Khadilkar R. Wagner’s classification as a tool for treating diabetic foot ulcers: our observations at a suburban teaching hospital. Cureus 2022; 14(1):e21501. doi: 10.7759/cureus.21501 [Crossref] [ Google Scholar]
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe Baseline results from the Eurodiale study. Diabetologia 2007; 50(1):18-25. doi: 10.1007/s00125-006-0491-1 [Crossref] [ Google Scholar]
- Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW. Diagnosis and treatment of diabetic foot infections. Plast Reconstr Surg 2006; 117(7 Suppl):212S-38S. doi: 10.1097/01.prs.0000222737.09322.77 [Crossref] [ Google Scholar]
- Shohrati M, Karimzadeh I, Saburi A, Khalili H, Ghanei M. The role of N-acetylcysteine in the management of acute and chronic pulmonary complications of sulfur mustard: a literature review. Inhal Toxicol 2014; 26(9):507-23. doi: 10.3109/08958378.2014.920439 [Crossref] [ Google Scholar]
- Jeremias A, Soodini G, Gelfand E, Xu Y, Stanton RC, Horton ES. Effects of N-acetyl-cysteine on endothelial function and inflammation in patients with type 2 diabetes mellitus. Heart Int 2009; 4(1):e7. doi: 10.4081/hi.2009.e7 [Crossref] [ Google Scholar]
- Panahi Y, Ghanei M, Mozaffari Hashjin M, Rezaee R, Sahebkar A. Potential utility of N-acetylcysteine for treating mustard lung. Crit Rev Eukaryot Gene Expr 2017; 27(3):247-66. doi: 10.1615/CritRevEukaryotGeneExpr.2017019740 [Crossref] [ Google Scholar]
- Eroshenko D, Polyudova T, Korobov V. N-acetylcysteine inhibits growth, adhesion and biofilm formation of gram-positive skin pathogens. Microb Pathog 2017; 105:145-52. doi: 10.1016/j.micpath.2017.02.030 [Crossref] [ Google Scholar]
- Manoharan A, Das T, Whiteley GS, Glasbey T, Kriel FH, Manos J. The effect of N-acetylcysteine in a combined antibiofilm treatment against antibiotic-resistant Staphylococcus aureus. J Antimicrob Chemother 2020; 75(7):1787-98. doi: 10.1093/jac/dkaa093 [Crossref] [ Google Scholar]
- Manoharan A, Ognenovska S, Paino D, Whiteley G, Glasbey T, Kriel FH. N-acetylcysteine protects bladder epithelial cells from bacterial invasion and displays antibiofilm activity against urinary tract bacterial pathogens. Antibiotics (Basel) 2021; 10(8):900. doi: 10.3390/antibiotics10080900 [Crossref] [ Google Scholar]
- Ahmed S, Darouiche RO. Anti-biofilm agents in control of device-related infections. Adv Exp Med Biol 2015; 831:137-46. doi: 10.1007/978-3-319-09782-4_9 [Crossref] [ Google Scholar]
- Janeczek M, Moy L, Riopelle A, Vetter O, Reserva J, Tung R. The potential uses of N-acetylcysteine in dermatology: a review. J Clin Aesthet Dermatol 2019; 12(5):20-6. [ Google Scholar]
- Aktunc E, Ozacmak VH, Ozacmak HS, Barut F, Buyukates M, Kandemir O. N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin Exp Dermatol 2010; 35(8):902-9. doi: 10.1111/j.1365-2230.2010.03823.x [Crossref] [ Google Scholar]
- Lavery LA, Ahn J, Ryan EC, Bhavan K, Oz OK, La Fontaine J. What are the optimal cutoff values for ESR and CRP to diagnose osteomyelitis in patients with diabetes-related foot infections?. Clin Orthop Relat Res 2019; 477(7):1594-602. doi: 10.1097/corr.0000000000000718 [Crossref] [ Google Scholar]
- Aragón-Sánchez J, Víquez-Molina G, López-Valverde ME, Aragón-Hernández J, Rojas-Bonilla JM, Murillo-Vargas C. Clinical, microbiological and inflammatory markers of severe diabetic foot infections. Diabet Med 2021; 38(10):e14648. doi: 10.1111/dme.14648 [Crossref] [ Google Scholar]
- Ong E, Farran S, Salloum M, Gardner S, Giovinco N, Armstrong D. The role of inflammatory markers: WBC, CRP, ESR, and neutrophil-to-lymphocyte ratio (NLR) in the diagnosis and management of diabetic foot infections. Open Forum Infect Dis 2015; 2(Suppl 1):1526. doi: 10.1093/ofid/ofv133.1079 [Crossref] [ Google Scholar]
- Wyman M, Dargan D, Kazzazi D, Caddick J, Giblin V. Serum inflammatory markers and amputations in hand osteomyelitis: a retrospective review of 146 cases. Hand (N Y) 2023; 18(6):987-93. doi: 10.1177/15589447211066346 [Crossref] [ Google Scholar]
- Williams JR. The Declaration of Helsinki and public health. Bull World Health Organ 2008; 86(8):650-2. doi: 10.2471/blt.08.050955 [Crossref] [ Google Scholar]
- Noorsyawala R, Yusufa FJ, Dahlan K, Dewib RM. PEDIS classification in diabetic foot ulcers patients. J Indones Soc Vasc Endovasc Surg 2020; 1(2):50-4. doi: 10.36864/jinasvs.2020.2.012 [Crossref] [ Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention,
and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59. doi: 10.1016/j.kisu.2017.04.001.
- Lamarche C, Iliuta IA, Kitzler T. Infectious disease risk in dialysis patients: a transdisciplinary approach. Can J Kidney Health Dis 2019; 6:2054358119839080. doi: 10.1177/2054358119839080 [Crossref] [ Google Scholar]
- Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci 2018; 1411(1):153-65. doi: 10.1111/nyas.13569 [Crossref] [ Google Scholar]
- Oguz A, Uslukaya O, Alabalık U, Turkoglu A, Kapan M, Bozdag Z. Topical N-acetylcysteine improves wound healing comparable to dexpanthenol: an experimental study. Int Surg 2015; 100(4):656-61. doi: 10.9738/intsurg-d-14-00227.1 [Crossref] [ Google Scholar]
- Zhang Q, Ju Y, Ma Y, Wang T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: a randomized controlled trial. Medicine (Baltimore) 2018; 97(45):e13087. doi: 10.1097/md.0000000000013087 [Crossref] [ Google Scholar]
- De Flora S, Balansky R, La Maestra S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J 2020; 34(10):13185-93. doi: 10.1096/fj.202001807 [Crossref] [ Google Scholar]
- Black PN, Morgan-Day A, McMillan TE, Poole PJ, Young RP. Randomised, controlled trial of N-acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med 2004; 4:13. doi: 10.1186/1471-2466-4-13 [Crossref] [ Google Scholar]
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30(2):239-45. doi: 10.1038/clpt.1981.154 [Crossref] [ Google Scholar]
- Baumgartner PC, Haynes RB, Hersberger KE, Arnet I. A systematic review of medication adherence thresholds dependent of clinical outcomes. Front Pharmacol 2018; 9:1290. doi: 10.3389/fphar.2018.01290 [Crossref] [ Google Scholar]
- Dinicola S, De Grazia S, Carlomagno G, Pintucci JP. N-acetylcysteine as powerful molecule to destroy bacterial biofilms A systematic review. Eur Rev Med Pharmacol Sci 2014; 18(19):2942-8. [ Google Scholar]
- Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CH, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep 2018; 8(1):10566. doi: 10.1038/s41598-018-28646-w [Crossref] [ Google Scholar]
- Li Y, Wang W, Yang F, Xu Y, Feng C, Zhao Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun Signal 2019; 17(1):147. doi: 10.1186/s12964-019-0471-y [Crossref] [ Google Scholar]
- Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio?. BMC Res Notes 2017; 10(1):12. doi: 10.1186/s13104-016-2335-5 [Crossref] [ Google Scholar]
- Yapıcı O, Berk H, Öztoprak N, Seyman D, Tahmaz A, Merdin A. Can ratio of neutrophil-to-lymphocyte count and erythrocyte sedimentation rate in diabetic foot infecti on predict osteomyelitis and/or amputation?. Hematol Rep 2017; 9(1):6981. doi: 10.4081/hr.2017.6981 [Crossref] [ Google Scholar]
- Sathvik M, Vuppuluri K, Dulipala P. The association of the neutrophil-lymphocyte ratio with the outcome of diabetic foot ulcer. Cureus 2023; 15(1):e33891. doi: 10.7759/cureus.33891 [Crossref] [ Google Scholar]
- Altay FA, Kuzi S, Altay M, Ateş İ, Gürbüz Y, Tütüncü EE. Predicting diabetic foot ulcer infection using the neutrophil-to-lymphocyte ratio: a prospective study. J Wound Care 2019; 28(9):601-7. doi: 10.12968/jowc.2019.28.9.601 [Crossref] [ Google Scholar]
- Michail M, Jude E, Liaskos C, Karamagiolis S, Makrilakis K, Dimitroulis D. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds 2013; 12(2):94-9. doi: 10.1177/1534734613486152 [Crossref] [ Google Scholar]
- Ertugrul BM, Savk O, Ozturk B, Cobanoglu M, Oncu S, Sakarya S. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit 2009; 15(6):CR307-12. [ Google Scholar]
- Fleischer AE, Didyk AA, Woods JB, Burns SE, Wrobel JS, Armstrong DG. Combined clinical and laboratory testing improves diagnostic accuracy for osteomyelitis in the diabetic foot. J Foot Ankle Surg 2009; 48(1):39-46. doi: 10.1053/j.jfas.2008.09.003 [Crossref] [ Google Scholar]
- van Asten SA, Jupiter DC, Mithani M, La Fontaine J, Davis KE, Lavery LA. Erythrocyte sedimentation rate and C-reactive protein to monitor treatment outcomes in diabetic foot osteomyelitis. Int Wound J 2017; 14(1):142-8. doi: 10.1111/iwj.12574 [Crossref] [ Google Scholar]
- van Asten SA, Nichols A, La Fontaine J, Bhavan K, Peters EJ, Lavery LA. The value of inflammatory markers to diagnose and monitor diabetic foot osteomyelitis. Int Wound J 2017; 14(1):40-5. doi: 10.1111/iwj.12545 [Crossref] [ Google Scholar]
- Durmaz B, Yilmaz S, Derebasinlioglu H. The role of inflammatory markers in the diagnosis and follow-up of diabetic foot osteomyelitis. Ann Med Res 2021; 27(4):1077-81. doi: 10.5455/annalsmedres.2019.11.694 [Crossref] [ Google Scholar]