Arch Iran Med. 27(9):501-507.
doi: 10.34172/aim.31133
Original Article
Histopathological Patterns of Lung Cancer in Iran: A Single-Center Study
Babak Salimi Conceptualization, Project administration, Writing – original draft, 1, # 
Sharareh Seifi Conceptualization, Writing – original draft, Writing – review & editing, 1, # 
Adnan Khosravi Funding acquisition, Methodology, 1
Sara Shiari Data curation, 1
Raana Moradi Data curation, 1
Babak Daneshfard Software, Writing – review & editing, 2, 3, 4
Maryam Mabani Conceptualization, Writing – review & editing, 1, * 
Author information:
1Research Center of Thoracic Oncology (RCTO), National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Science, Tehran, Iran
2Chronic Respiratory Diseases Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
3Persian Medicine Network (PMN), Universal Scientific Education and Research Network (USERN), Tehran, Iran
4Canadian College of Integrative Medicine, Montreal, Quebec, Canada
#Both authors contributed equally to this research.
Abstract
Background:
Lung cancer (LC) is one of the leading causes of cancer-related deaths worldwide. In Iran, it is the second most common cause of cancer-related deaths for men and the third most common for women. This study aimed to examine the clinicopathological characteristics of Iranian patients with LC.
Methods:
Clinicopathological data of 1382 patients with primary LC diagnosed over 11 years (2012‒2023) at the "National Institute of Tuberculosis and Lung Disease" (NRITLD), Tehran, Iran, were retrospectively reviewed.
Results:
Adenocarcinoma was the most common type of cancer found in the patients (42.44%). The median age was 59.69 years (mean: 60.41 years) ranging 24–88 years. The mean male-to-female ratio was 3.65. Additionally, 65.84% of patients were smokers. The majority of patients (82.69 %) were diagnosed at an advanced stage (stage IV) of cancer.
Conclusion:
Although some of our findings are consistent with those of previous LC studies, there are some discrepancies, especially concerning the smoking status and median age of the Iranian patients. Therefore, additional clinical and epidemiological studies are needed to determine the impact of non-smoking factors, such as environmental exposure and genetic predisposition, on the development of LC.
Keywords: Epidemiology, Iran, Lung cancer, Risk factor
Copyright and License Information
© 2024 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Salimi B, Seifi S, Khosravi A, Shiari S, Moradi R, Daneshfard B, et al. Histopathological patterns of lung cancer in Iran: a single-center study. Arch Iran Med. 2024;27(9):501-507. doi: 10.34172/aim.31133
Introduction
Lung cancer (LC) is the second most common type of cancer in both males and females and the leading cause of cancer-related deaths globally. Despite advances in treatment, the five-year survival rate remains at 19%, with 13% of all cancer cases and 24% of all cancer deaths attributable to LC.1 According to Garcia et al. (2007), the five-year survival rate for LC patients is only 15.9%.2 LC incidence is heavily influenced by geographic location, with higher rates observed in developed countries compared to underdeveloped ones. Moreover, the incidence of LC is on the rise in Asian countries.3 In Iran, LC is one of the most prevalent types of cancer, exhibiting an increasing trend and causing significant economic burdens. Its pattern varies across different geographical areas within Iran.4
LC morbidity and mortality are more prevalent among men.5 This is likely due to several interconnected risk factors including smoking, diet, occupational exposures, environmental risks, familial history, and gender. Among these, smoking stands out as the most significant risk factor for LC.6 Studies have demonstrated that changing trends in smoking,7 diet,8 and other environmental and lifestyle factors,9 including air pollution,10,11 and occupational exposures,10 significantly influence the incidence and mortality of LC in Iran. These variations are further observed within different gender and histopathological subgroups. Our study emphasizes the critical need for accurate and timely data on these dynamic risk factors to improve diagnosis, treatment strategies, and patient outcomes in the Iranian context.
This study investigates the epidemiological, pathological, and clinical attributes of 1832 primary LC cases in Iranian patients, who were diagnosed and treated at our specialized oncology center over a span of 11 years. Our objective is to uncover distinct patterns and features within this unique population, with the goal of enriching our knowledge of LC in Iran and subsequently informing more effective diagnosis and treatment strategies.
Materials and Methods
A retrospective, hospital-based cross-sectional study was conducted using data from 1,382 patients with primary LC diagnosed between August 2012 and June 2023 at the National Research Institute of Tuberculosis and Lung Disease (NRITLD). Patient diagnoses were confirmed by pathology specimens. NRITLD is an academic hospital affiliated with Shahid Beheshti University of Medical Sciences in Tehran, Iran. For this study, metastatic lung neoplasms originating from primary sites other than the lungs were excluded. Information on the patients’ demographic characteristics, smoking history, histological subtype, and cancer stage was obtained through chart reviews. Tissue specimens were categorized according to the 1981 World Health Organization (WHO) classification system for LC.12
Non-small-cell lung cancer, not otherwise specified (NSCLC-NOS), is a diagnosis given to patients whose cancer cells do not clearly fall under the categories of adenocarcinoma or squamous cell carcinoma (SCC). Adenocarcinoma, large cell carcinoma, small cell lung carcinoma (SCLC), and SCC are the four forms of primary LCs. For patients with SCLC, a different staging system was used. Limited disease is defined as cancer confined to the same side of the chest as the tumor and can be covered within a single radiotherapy port. This corresponds to TNM stages I–IIIB. On the other hand, extensive disease is defined as cancer that has spread beyond the ipsilateral hemithorax (the side of the chest where the tumor is located) and metastasized to other parts of the body.13 For this study, a non-smoker was defined as someone who had smoked fewer than 100 cigarettes in their lifetime.14 However, exposure to passive smoking was not evaluated.
A chi-square test was employed to assess the relationship between LC types and various factors, including age, gender, smoking status, and addiction. This analysis aimed to identify any statistically significant associations between these variables and the specific types of LC.
Furthermore, an analysis of variance (ANOVA) was conducted to compare the average age of patients diagnosed with different LC types. This test helped determine whether the observed differences in average age were statistically significant or simply due to chance.
A significance level of 5% was established for all statistical tests, and the analyses were conducted using SPSS version 27.
Results
The study included 1382 patients, of whom 1085 (78.45%, 60.41 ± 10.55) were men and 297 (21.47%, 57.06 ± 12.30) were women (male/female ratio: 3.65). The average age of the patients was 59.69 ± 11.03 years (60.41 years for men 57 years for women). The main characteristics of the patients are summarized in Table 1.
Table 1.
Prevalence of Common Morphologies of Lung Cancers in Patients Referred to Masih Deneshvari Hospital between 2012-2022
|
|
All (n=1382)
(21-88 y)
|
Male (n=1085)
(24-88 y)
|
Female (n=297)
(21-87 y)
|
P
Value
|
| Age of diagnosis (Mean ± SD) |
59.69 ± 11.03 |
60.41 ± 10.55 |
57.06 ± 12.30 |
0.0001 |
| Histology |
|
|
|
|
| Adenosquamous cell carcinoma |
2 (.14%) |
2 (100%) |
0 (0%) |
0.0001 |
| Not otherwise specified |
30 (2.16%) |
25 (83.3%) |
5 (16.7%) |
| Primary Adenocarcinoma |
587 (42.44%) |
403 (68.7%) |
184 (31.3%) |
| Small cell carcinoma |
319 (23.06%) |
275 (86.2%) |
44 (13.8%) |
| Squamous cell carcinoma |
444 (32.10%) |
380 (85.6%) |
64 (14.4%) |
| Total |
1382 (100) |
1085 (100) |
297 (100) |
| Disease stage in non-small cell carcinoma |
|
|
|
|
| I A |
1 (.09 %) |
1 (.12 %) |
0 (0 %) |
0.002 |
| I B |
2 (.18%) |
1 (.12 %) |
1 (.39 %) |
| II A |
29 (2.72 %) |
23 (2.84 %) |
6 (2.35 %) |
| II B |
5 (.47%) |
4 (.49 %) |
1 (.39 %) |
| III A |
75 (7.05 %) |
63 (7.79 %) |
12 (4.70%) |
| III B |
68 (6.39 %) |
51 (6.31%) |
17 (6.66%) |
| III C |
4 (.37 %) |
4 (.49%) |
0 (0 %) |
| IV A |
879 (82.69 %) |
661 (81.80 %) |
218 (85.49%) |
| Total |
1063 (100%) |
808 (100%) |
255 (100%) |
| Disease stage in small cell carcinoma |
|
|
|
|
| Limited stage |
78 (24.45 %) |
72 (26.08 %) |
6 (13.95 %) |
.058 |
| Extensive stage |
241 (75.54%) |
204 (73.91%) |
37 (86.04 %) |
| Total |
319(100%) |
276 (100 %) |
43 (100%) |
| Smoking status |
|
|
|
|
| Smoker |
910 (65.84%) |
857 (94.17 %) |
53 (5.82 %) |
0.0001 |
| Non-smoker |
472 (34.15%) |
228 (48.30 %) |
244(51.69 %) |
| Addicted status |
|
|
|
|
| Addicted |
859 (62.15 %) |
591 (54.52%) |
28 (8.53 %) |
0.0001 |
| Not addicted |
521 (37.69 %) |
493 (45.47 %) |
300 (91.46 %) |
The vast majority (76.84%) of LC cases belonged to the NSCLC category (60.03 ± 11.26), far exceeding the prevalence of SCLC at 23.06% (58.56 ± 10.16). This skewed distribution shown in Figure 1 has significant implications for LC diagnosis, treatment, and prognosis. Adenocarcinoma was the most common pathology in LC patients of both genders (Table 1). Upon closer inspection, there were notable distinctions in the diagnoses based on gender (Figure 2). SCC was the most common kind of presentation in men, whereas SCLC was the most common type in women (Figure 2). This disparity highlights the possibility that different underlying biological factors and treatment considerations exist for different genders. Age, histology, and NSCLC stage at diagnosis were shown to differ significantly between men and women (P = 0.001, 0.0001, and 0.002, respectively). Approximately, 62.15% of the patients were drug abusers, and 65.84% were smokers. Smoking and addiction were significantly more prevalent among men than women (P = 0.0001).
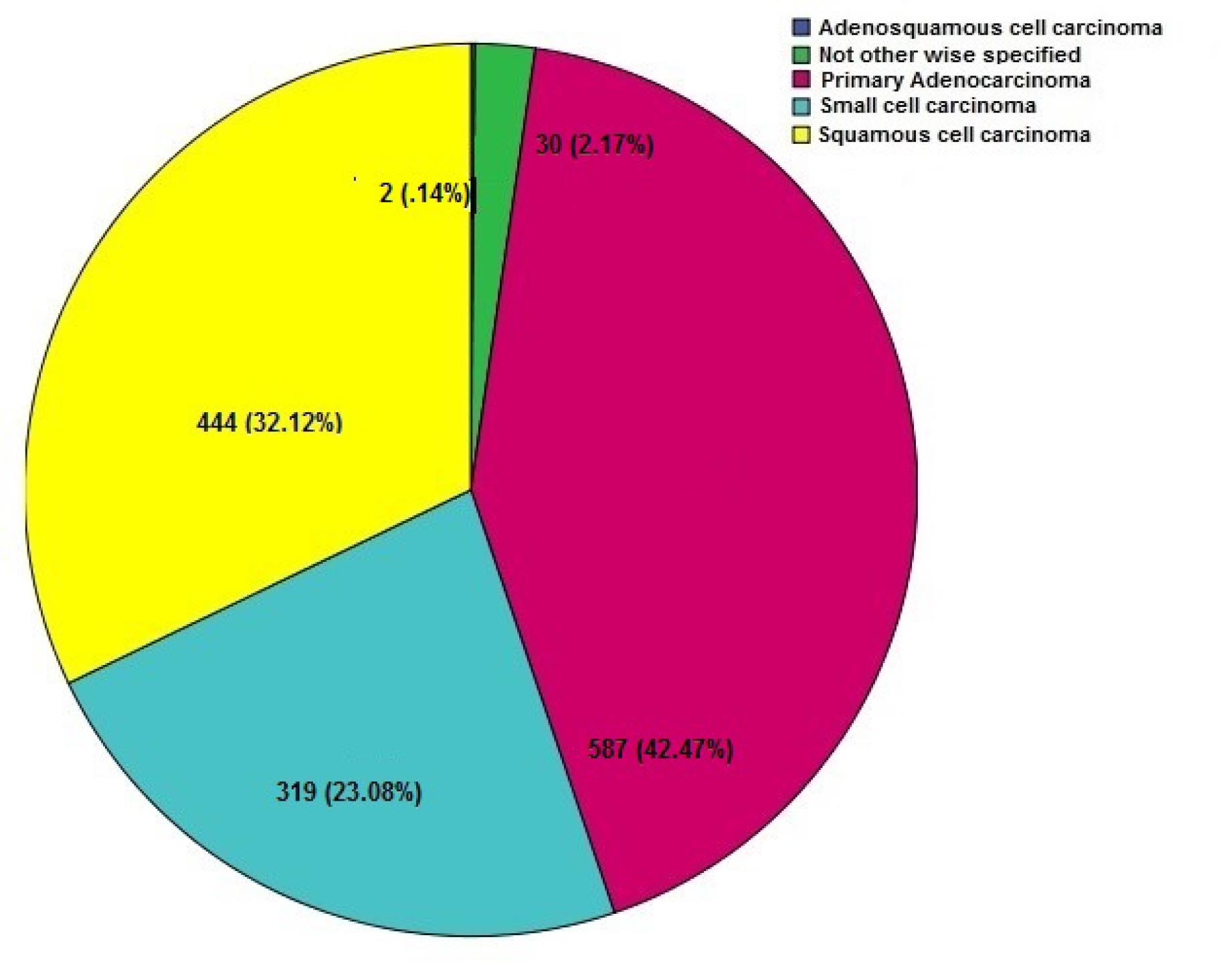
Figure 1.
Histopathology Subtypes (n = 1382)
.
Histopathology Subtypes (n = 1382)
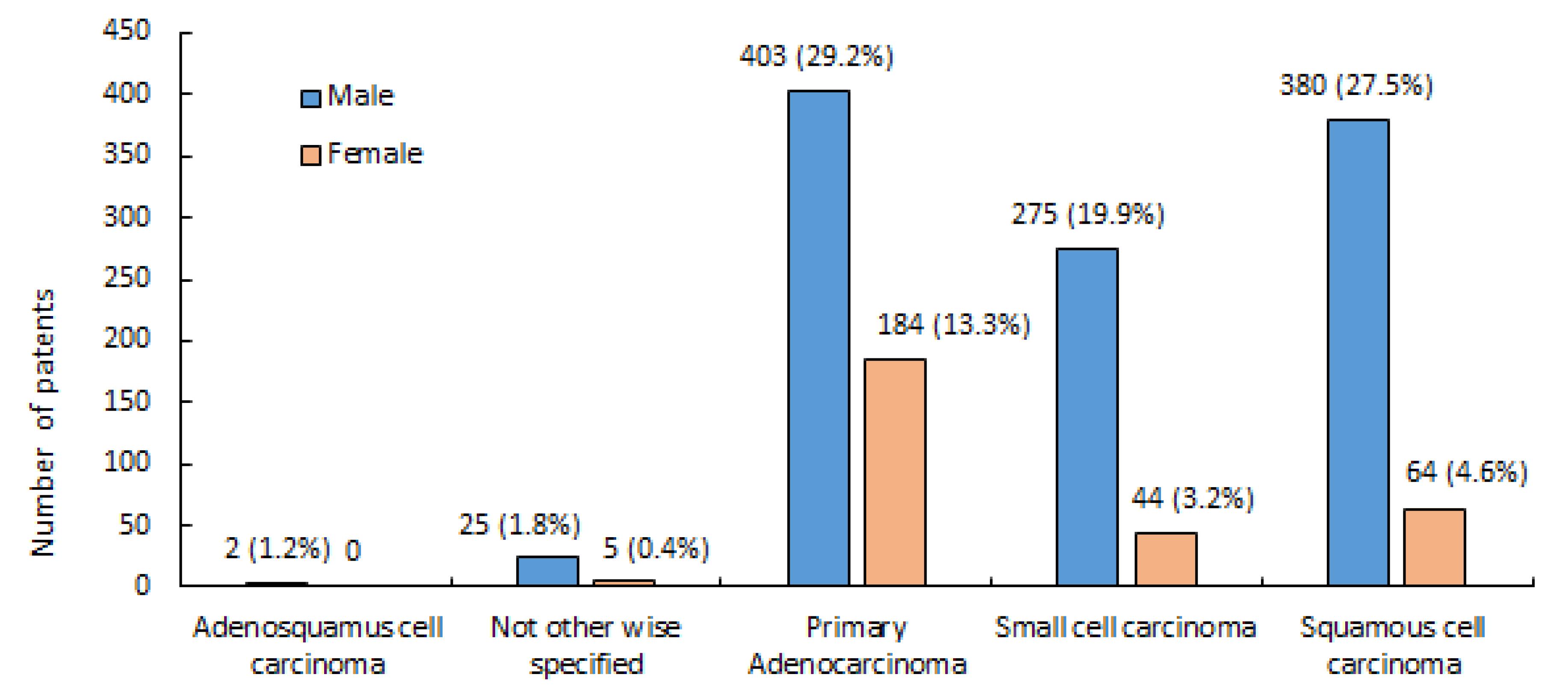
Figure 2.
Distribution According to Gender and Histological Subtypes
.
Distribution According to Gender and Histological Subtypes
The analysis presented in Table 2 emphasizes that age plays a vital role in distinguishing the various subtypes of LC. Notably, patients with SCC exhibited a significantly higher average age compared to those with NSCLC-NOS (P = 0.038), primary adenocarcinoma (P = 0.0001), and small cell carcinoma (P = 0.0001). Further exploration is necessary to examine the biological and clinical consequences of age differences in LC diagnoses.
Table 2.
Pairwise Comparison of Average Age in the Studied Groups
|
Cancer Type
|
Cancer Type
|
P
Value
|
| Adenosquamous cell carcinoma |
Not otherwise specified |
0.549 |
| Primary adenocarcinoma |
0.669 |
| Small cell carcinoma |
0.696 |
| Squamous cell carcinoma |
0.924 |
| Not otherwise specified |
Adenosquamous cell carcinoma |
0.549 |
| Primary adenocarcinoma |
0.910 |
| Small cell carcinoma |
0.855 |
| Squamous cell carcinoma |
0.038 |
| Primary Adenocarcinoma |
Adenosquamous cell carcinoma |
0.669 |
| Not otherwise specified |
0.910 |
| Small cell carcinoma |
0.994 |
| Squamous cell carcinoma |
0.000 |
| Small cell carcinoma |
Adenosquamous cell carcinoma |
0.696 |
| Not otherwise specified |
0.855 |
| Primary adenocarcinoma |
0.994 |
| Squamous cell carcinoma |
0.000 |
| Squamous cell carcinoma |
Adenosquamous cell carcinoma |
0.924 |
| Not otherwise specified |
0.038 |
| Primary adenocarcinoma |
0.000 |
| Small cell carcinoma |
0.000 |
Table 3 shows the relative risk or odds ratio, along with 95% confidence intervals, for the relationship between smoking, addiction, age, and different types of LC. In the case of smoking, the incidence of not otherwise specified types of cancer was higher than that of the other types. In addition, the ratio of primary adenocarcinomas in smokers was 4.9 times higher than that of small cell carcinomas and 2.6 times higher than that of SCCs. The proportion of SCC was 1.9 times higher than that of small-cell carcinoma.
Table 3.
Odds Ratio with a 95% Confidence Interval, Relating to the relationship Between Age, Smoking, Addiction, and Types of Lung Cancer
|
Cancer Type
|
Cancer Type
|
OR
Age
|
OR
Addicted
|
OR Smoke
|
| Adenosquamous cell carcinoma |
Not otherwise specified |
1.161 |
- |
- |
| Primary adenocarcinoma |
1.130 |
- |
- |
| Small cell carcinoma |
1.138 |
- |
- |
| Squamous cell carcinoma |
1.080 |
- |
- |
| Not otherwise specified |
Adenosquamous cell carcinoma |
0.861 |
- |
- |
| Primary adenocarcinoma |
0.982 |
0.792 |
1.324* |
| Small cell carcinoma |
0.980 |
1.320 |
6.133* |
| Squamous cell carcinoma |
0.953* |
0.763 |
2.698* |
| Primary Adenocarcinoma |
Adenosquamous cell carcinoma |
0.885 |
- |
- |
| Not otherwise specified |
1.018 |
1.263 |
0.755* |
| Small cell carcinoma |
1.002 |
1.324 |
4.978* |
| Squamous cell carcinoma |
0.970* |
0.869 |
2.690* |
| Small cell carcinoma |
Adenosquamous cell carcinoma |
0.878 |
- |
- |
| Not otherwise specified |
1.021 |
0.758 |
0.163* |
| Primary adenocarcinoma |
0.998 |
0.755 |
0.201* |
| Squamous cell carcinoma |
0.960* |
0.687* |
0.501* |
| Squamous cell carcinoma |
Adenosquamous cell carcinoma |
0.926 |
- |
- |
| Not otherwise specified |
1.049* |
1.310* |
0.371* |
| Primary Adenocarcinoma |
1.031* |
1.151 |
0.372* |
| Small cell carcinoma |
1.042* |
1.456* |
1.996* |
* The mean difference is significant at the 0.05 level.
In terms of age, the proportion of squamous cell cancers was higher than that of other types of LCs.
In addition, the proportion of drug-related SCC was 1.3 and 1.4 times higher than those classified as “not otherwise specified” and small cell carcinoma, respectively.
Discussion
This study examined the demographic and clinicopathological characteristics of a cohort of Iranian LC patients. It aims to address the lack of comprehensive studies on LC in Iran that involve a large number of patients from a single institution. By utilizing data from 1382 patients, the study provides valuable insight into the regional trends and difficulties associated with this disease, which enhances our knowledge of the condition in this specific context. Our study reveals a notable difference in the diagnosis of LC between genders. Despite the average age of 59.69 years for the group, men have a significantly higher median age of 60.41 years as compared to women with an average age of 57 years (P < 0.05).
It is important to note that adenocarcinoma was the most common type of LC, with males being affected 3.65 times more than females. Our research also reveals a significant difference in smoking habits, with men having a much higher prevalence of smoking at 94% compared to women at only 5%. Additionally, a staggering 76.84% of the patients in our study were diagnosed with advanced-stage LC.
Refining early detection strategies and implementing gender-specific approaches are urgent to address the critical risk factor of age in cancer incidence. LC incidence is low before the age of 50 and then increases noticeably.14,15 This trend reflects the impact of aging populations in developed countries, where cancer disproportionately affects older people.16 Our study found that the average age of diagnosis for participants was 59.69 years, consistent with previous studies in Iran,17-19 Turkey (60 years),20 and India (56 years).21 It is worth noting that the age at which a diagnosis is made can vary based on geographical location. For instance, in Arab countries like Saudi Arabia, Kuwait, and the UAE, the average age at diagnosis is higher, at around 66 years.22 Similarly, developed countries like Canada (75 years),23 the USA (74 years),24 Japan (66.3 years),25 and Australia (71 years) also show a higher average age at diagnosis.26 The differences in racial/ethnic compositions may explain the observed variations in the mean age at diagnosis. Research conducted by Hamid et al27 suggests that these racial/ethnic characteristics might play a mediating role through genetic and lifestyle interactions. Nonetheless, it is crucial to consider other potential explanations, as well.
It is possible that the differences in the average age of diagnosis of LC in Iran could be due to the high number of young smokers who died from cardiovascular diseases in the past. The improvements in cardiovascular care may have increased the survival rates of these individuals, which could have led to more cases of LC being diagnosed at a later stage in life. This could explain the observed increase in both incidence and median age at diagnosis in developing countries like Iran.27
In our study, we found that women were diagnosed with LC at a younger age than men, which is consistent with previous research.14,28-31 Interestingly, despite being more likely to be lifetime non-smokers with shorter smoking histories and smoking fewer cigarettes per day, this disparity remained consistent among women in our study.13,32,33 Recent studies suggest that there may be other risk factors apart from smoking that contribute to the development of LC in women. Previous investigations have suggested that women’s genetic profile may increase their vulnerability to the carcinogenic effects of cigarette smoke and environmental toxins.34
Significant disparities exist in the male/female ratio across different countries. Our study found a ratio of 3.65, consistent with prior researches in Iran (with a range of 2.79 to 5.09),35 Kuwait,36 and Japan.37 Certain countries, like Spain (which reported a ratio of 8.1)38 and India,39 have reported higher male/female ratios than others. Discrepancies in these ratios may be influenced by various factors, including smoking status, DNA repair capabilities, histological subtypes, and alcohol consumption.40
There is a possibility that the disparity in LC incidence between men and women may be traced back to historical differences in smoking habits.5 Studies indicate that smoking is a major contributor to LC, responsible for 80%‒91% of cases in men and 45%‒69% of cases in women.41,42
Even though smoking is the primary reason for LC, it is estimated that approximately a quarter of LC patients across the globe have never smoked.14 Non-smoking LC patients are mostly women with adenocarcinoma and a younger age of onset.43 Notably, the smoking prevalence in Iran fluctuates between 23.9% and 26% in men and 1.7% and 3.6% in women.44,45
In Iran, social stigma associated with smoking in women might be the reason why only 5.82% of the 297 females were smokers in a recent study. Our study highlights the fact that LC is influenced by a combination of genetic and environmental factors. Another study found that LC in Asian women who have never smoked is associated with variations in three genomic locations on chromosomes 6 and 10. Asian women may be more vulnerable to the effects of second-hand smoke.46
Certain genes, such as GRPR, are expressed more frequently in non-smoking women than in men and have been linked to bronchial cell proliferation.47 Similarly, polymorphisms in the ER gene have been connected to lung adenocarcinoma in women who have never smoked. These observations suggest that genetic factors may play a role in the development of LC, regardless of smoking habits.48
In recent years, the incidence of adenocarcinoma has surpassed that of SCC.49 This may be due to changes in cigarette composition and smoking method.50 Technological advancements have shifted cases from large cell carcinoma to adenocarcinoma due to improvements in identifying peripheral pulmonary lesions, modifications in the WHO classification, and enhancements in staining mucin-producing cells.51 Another factors that might contribute to the increase in adenocarcinoma cases is air pollution, particularly nitrogen oxides.52
The most frequently observed pathology was adenocarcinoma, which is consistent with previous research.17,53,54 In our study, the majority of patients were at advanced stages, which is consistent with international reports.55
While this retrospective study offers valuable insight, it is important to recognize its limitations. Firstly, the accuracy of the smoking data might be compromised due to its retrospective nature. Secondly, we were unable to assess the impact of other potential contributing factors, such as passive smoking, air pollution, or substances like silica or asbestos. Moreover, studies suggest that individuals with a family history of LC diagnosed before the age of 60 are at a higher risk of developing the disease.56 However, it was not possible to investigate the family history of the patients in this study as the available data was incomplete.
There are differing opinions regarding the role of infection as a causative agent for LC. Some researchers believe that certain viruses, such as human papillomavirus,57 Epstein-Barr virus,58 human cytomegalovirus, simian virus 40 (SV40), and measles virus, are associated with LC.9 Pulmonary tuberculosis, a common infection in Iran, has also been associated with LC.59 It is important to conduct further studies to determine the association between different infections and LC across various geographic regions. This study was carried out on patients who were referred to the tertiary center of NRTLD from all over Iran. However, it is important to note that the findings may not precisely represent the clinicopathologic and demographic characteristics of all Iranian patients diagnosed with LC.
Conclusion
The clinicopathological features of Iranian LC patients are highlighted in this research, revealing potential differences in patterns and triggers between developing nations and developed countries. Our research is in line with studies carried out in regional or developing countries. It is worth mentioning that our study discovered that patients diagnosed with LC in Iran were younger and had a lower rate of smoking compared to other studies. This variation could be due to other factors, such as genetic predisposition, endemic infectious diseases, and environmental exposures. In order to gain a better understanding of the link between smoking and LC in developing countries, it is essential to establish a thorough national cancer registry. This would enable the sharing of data and resources, promote research, and aid the development of effective strategies to combat LC, through regional and international cooperation.
Acknowledgements
We are deeply indebted to the participating investigators, Drs. Farnia, and Khosravi, whose expertise and tireless efforts guided this research. The clinical staff at Masih Daneshvari Hospital deserve our highest praise for their meticulous data collection and patient care, which ensured the study’s integrity and success. We express our heartfelt gratitude to the patients and their families for their courage and trust in entrusting us with their health data. This work was supported by a grant from the National Research Institute of Tuberculosis and Lung Diseases.
Competing Interests
The authors declare that they have no conflict of interest.
Ethical Approval
All participants provided informed consent after receiving detailed information about the study. Data were collected anonymously to protect participant privacy. The authors declare no conflicts of interest. Animal studies were not conducted. The data will be made available upon request to qualified researchers.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69(1):7-34. doi: 10.3322/caac.21551 [Crossref] [ Google Scholar]
- Kim RB, Phillips A, Herrick K, Helou M, Rafie C, Anscher MS. Physical activity and sedentary behavior of cancer survivors and non-cancer individuals: results from a national survey. PLoS One 2013; 8(3):e57598. doi: 10.1371/journal.pone.0057598 [Crossref] [ Google Scholar]
- Wong MC, Lao XQ, Ho KF, Goggins WB, Tse SL. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep 2017; 7(1):14300. doi: 10.1038/s41598-017-14513-7 [Crossref] [ Google Scholar]
- Roshandel G, Ghanbari-Motlagh A, Partovipour E, Salavati F, Hasanpour-Heidari S, Mohammadi G. Cancer incidence in Iran in 2014: results of the Iranian National Population-based Cancer Registry. Cancer Epidemiol 2019; 61:50-8. doi: 10.1016/j.canep.2019.05.009 [Crossref] [ Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65(1):5-29. doi: 10.3322/caac.21254 [Crossref] [ Google Scholar]
- Sadeghi-Gandomani H, Asgari-Tarazoj A, Ghoncheh M, Yousefi SM, Delaram M, Salehiniya H. Lung cancer in the world: the incidence, mortality rate and risk factors. World Cancer Res J 2017; 4:e911. [ Google Scholar]
- Couraud S, Zalcman G, Milleron B, Morin F, Souquet PJ. Lung cancer in never smokers--a review. Eur J Cancer 2012; 48(9):1299-311. doi: 10.1016/j.ejca.2012.03.007 [Crossref] [ Google Scholar]
- Ruano-Ravina A, Figueiras A, Freire-Garabal M, Barros-Dios JM. Antioxidant vitamins and risk of lung cancer. Curr Pharm Des 2006; 12(5):599-613. doi: 10.2174/138161206775474396 [Crossref] [ Google Scholar]
- Gilliland FD, Hunt WC, Archer VE, Saccomanno G. Radon progeny exposure and lung cancer risk among non-smoking uranium miners. Health Phys 2000; 79(4):365-72. doi: 10.1097/00004032-200010000-00004 [Crossref] [ Google Scholar]
- Malhotra J, Sartori S, Brennan P, Zaridze D, Szeszenia-Dabrowska N, Świątkowska B. Effect of occupational exposures on lung cancer susceptibility: a study of gene-environment interaction analysis. Cancer Epidemiol Biomarkers Prev 2015; 24(3):570-9. doi: 10.1158/1055-9965.epi-14-1143-t [Crossref] [ Google Scholar]
- Hoek G, Raaschou-Nielsen O. Impact of fine particles in ambient air on lung cancer. Chin J Cancer 2014; 33(4):197-203. doi: 10.5732/cjc.014.10039 [Crossref] [ Google Scholar]
- World Health Organization. The World Health Organization histological typing of lung tumours Second edition. Am J Clin Pathol 1982; 77(2):123-36. doi: 10.1093/ajcp/77.2.123 [Crossref] [ Google Scholar]
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007; 2(8):706-14. doi: 10.1097/JTO.0b013e31812f3c1a [Crossref] [ Google Scholar]
- Wender R, Fontham ET, Barrera E Jr, Colditz GA, Church TR, Ettinger DS. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013; 63(2):107-17. doi: 10.3322/caac.21172 [Crossref] [ Google Scholar]
- Radzikowska E, Głaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival Population-based study of 20 561 cases. Ann Oncol 2002; 13(7):1087-93. doi: 10.1093/annonc/mdf187 [Crossref] [ Google Scholar]
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011; 32(4):605-44. doi: 10.1016/j.ccm.2011.09.001 [Crossref] [ Google Scholar]
- Mehrbani D, Tabeei SZ, Heydari ST, Shamsina SJ, Shokrpour N, Amini M. Cancer occurrence in Fars province, southern Iran. Iran Red Crescent Med J 2008; 10(4):314-22. [ Google Scholar]
- Adnan K, Esfahani-Monfared Z, Sei S, Karimi S, Emami H, Khodadad K. Clinicopathological characteristics of Iranian patients with lung cancer: a single institute experience. Asian Pac J Cancer Prev 2016; 17(8):3817-22. [ Google Scholar]
- Hashemi Sadraei N, Riahi T, Masjedi MR. Idiopathic pulmonary fibrosis in a referral center in Iran: are patients developing the disease at a younger age?. Arch Iran Med 2013; 16(3):177-81. [ Google Scholar]
- Gonlugur U, Gonlugur TE, Kaptanoglu M, Nadir A, Cinar Z. The changing epidemiological trends for carcinoma of the lung in Turkey. Saudi Med J 2008; 29(5):749-53. [ Google Scholar]
- Noronha V, Dikshit R, Raut N, Joshi A, Pramesh CS, George K. Epidemiology of lung cancer in India: focus on the differences between non-smokers and smokers: a single-centre experience. Indian J Cancer 2012; 49(1):74-81. doi: 10.4103/0019-509x.98925 [Crossref] [ Google Scholar]
- Al-Hashimi MM, Wang XJ. Trend analysis of lung cancer incidence rates in Ninawa province, Iraq, from 2000 to 2010--decrease and recent stability. Asian Pac J Cancer Prev 2014; 15(1):385-90. doi: 10.7314/apjcp.2014.15.1.385 [Crossref] [ Google Scholar]
- Navaneelan T, Janz T. Cancer in Canada: Focus on Lung, Colorectal, Breast and Prostate. Statistics Canada; 2011.
- Zacharia BE, Bruce SS, Goldstein H, Malone HR, Neugut AI, Bruce JN. Incidence, treatment and survival of patients with craniopharyngioma in the surveillance, epidemiology and end results program. Neuro Oncol 2012; 14(8):1070-8. doi: 10.1093/neuonc/nos142 [Crossref] [ Google Scholar]
- Osawa K, Nakarai C, Uchino K, Yoshimura M, Tsubota N, Takahashi J. XRCC3 gene polymorphism is associated with survival in Japanese lung cancer patients. Int J Mol Sci 2012; 13(12):16658-67. doi: 10.3390/ijms131216658 [Crossref] [ Google Scholar]
- Cheng ES, Weber M, Feletto E, Smith MA, Yu XQ. Cancer burden and control in Australia: lessons learnt and challenges remaining. Ann Cancer Epidemiol 2018; 2:3. doi: 10.21037/ace.20 [Crossref] [ Google Scholar]
- Hamid MS, Shameem R, Gafoor K, George J, Mina B, Sullivan K. Non-small-cell lung cancer clinicopathologic features and survival outcomes in Asian Pacific Islanders residing in the United States: a SEER analysis. J Cancer Epidemiol 2015; 2015:269304. doi: 10.1155/2015/269304 [Crossref] [ Google Scholar]
- Minami H, Yoshimura M, Miyamoto Y, Matsuoka H, Tsubota N. Lung cancer in women: sex-associated differences in survival of patients undergoing resection for lung cancer. Chest 2000; 118(6):1603-9. doi: 10.1378/chest.118.6.1603 [Crossref] [ Google Scholar]
- Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest 2005; 128(1):370-81. doi: 10.1378/chest.128.1.370 [Crossref] [ Google Scholar]
- de Perrot M, Licker M, Bouchardy C, Usel M, Robert J, Spiliopoulos A. Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J Thorac Cardiovasc Surg 2000; 119(1):21-6. doi: 10.1016/s0022-5223(00)70213-3 [Crossref] [ Google Scholar]
- Baldini EH, Strauss GM. Women and lung cancer: waiting to exhale. Chest 1997; 112(4 Suppl):229S-34S. doi: 10.1378/chest.112.4_supplement.229s [Crossref] [ Google Scholar]
- Fontham ET, Correa P, Reynolds P, Wu-Williams A, Buffler PA, Greenberg RS. Environmental tobacco smoke and lung cancer in nonsmoking women A multicenter study. JAMA 1994; 271(22):1752-9. [ Google Scholar]
- Wang TJ, Zhou BS, Shi JP. Lung cancer in nonsmoking Chinese women: a case-control study. Lung Cancer 1996; 14 Suppl 1:S93-8. doi: 10.1016/s0169-5002(96)90214-7 [Crossref] [ Google Scholar]
- Koo LC, Ho JH. Worldwide epidemiological patterns of lung cancer in nonsmokers. Int J Epidemiol 1990; 19 Suppl 1:S14-23. doi: 10.1093/ije/19.supplement_1.s14 [Crossref] [ Google Scholar]
- Hajmanoochehri F, Mohammadi N, Zohal MA, Sodagar A, Ebtehaj M. Epidemiological and clinicopathological characteristics of lung cancer in a teaching hospital in Iran. Asian Pac J Cancer Prev 2014; 15(6):2495-500. doi: 10.7314/apjcp.2014.15.6.2495 [Crossref] [ Google Scholar]
- El-Basmy A. Profile of lung cancer in Kuwait. Asian Pac J Cancer Prev 2013; 14(10):6181-4. doi: 10.7314/apjcp.2013.14.10.6181 [Crossref] [ Google Scholar]
- Kanematsu T, Hanibuchi M, Tomimoto H, Sakiyakma S, Kenzaki K, Kondo K. Epidemiological and clinical features of lung cancer patients from 1999 to 2009 in Tokushima Prefecture of Japan. J Med Invest 2010; 57(3-4):326-33. doi: 10.2152/jmi.57.326 [Crossref] [ Google Scholar]
- Santos-Martínez MJ, Curull V, Blanco ML, Macià F, Mojal S, Vila J. [Lung cancer at a university hospital: epidemiological and histological characteristics of a recent and a historical series]. Arch Bronconeumol 2005; 41(6):307-12. doi: 10.1016/s1579-2129(06)60230-9.[Spanish] [Crossref] [ Google Scholar]
- Dey A, Biswas D, Saha SK, Kundu S, Kundu S, Sengupta A. Comparison study of clinicoradiological profile of primary lung cancer cases: an Eastern India experience. Indian J Cancer 2012; 49(1):89-95. doi: 10.4103/0019-509x.98930 [Crossref] [ Google Scholar]
- Chang JS, Wrensch MR, Hansen HM, Sison JD, Aldrich MC, Quesenberry CP Jr. Base excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African-Americans. Carcinogenesis 2009; 30(1):78-87. doi: 10.1093/carcin/bgn261 [Crossref] [ Google Scholar]
- Salim EI, Jazieh AR, Moore MA. Lung cancer incidence in the Arab league countries: risk factors and control. Asian Pac J Cancer Prev 2011; 12(1):17-34. [ Google Scholar]
- Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer 2004; 45 Suppl 2:S3-9. doi: 10.1016/j.lungcan.2004.07.998 [Crossref] [ Google Scholar]
- Ou SH, Ziogas A, Zell JA. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol 2009; 4(9):1083-93. doi: 10.1097/JTO.0b013e3181b27b15 [Crossref] [ Google Scholar]
- Ahmadi J, Khalili H, Jooybar R, Namazi N, Mohammadagaei P. Prevalence of cigarette smoking in Iran. Psychol Rep 2001; 89(2):339-41. doi: 10.2466/pr0.2001.89.2.339 [Crossref] [ Google Scholar]
- Yunesian M, Homayoun-Vash J, Asghari F, Foruzanfar MH, Hosein-Poor AR, Farhud D. Smoking-related respiratory symptoms in Tehran: a cross-sectional study. Arch Iran Med 2008; 11(5):507-14. [ Google Scholar]
- Lan Q, Hsiung CA, Matsuo K, Hong YC, Seow A, Wang Z. Genome-wide association analysis identifies new lung cancer susceptibility loci in never-smoking women in Asia. Nat Genet 2012; 44(12):1330-5. doi: 10.1038/ng.2456 [Crossref] [ Google Scholar]
- Shriver SP, Bourdeau HA, Gubish CT, Tirpak DL, Davis AL, Luketich JD. Sex-specific expression of gastrin-releasing peptide receptor: relationship to smoking history and risk of lung cancer. J Natl Cancer Inst 2000; 92(1):24-33. doi: 10.1093/jnci/92.1.24 [Crossref] [ Google Scholar]
- Chen KY, Hsiao CF, Chang GC, Tsai YH, Su WC, Chen YM. Estrogen receptor gene polymorphisms and lung adenocarcinoma risk in never-smoking women. J Thorac Oncol 2015; 10(10):1413-20. doi: 10.1097/jto.0000000000000646 [Crossref] [ Google Scholar]
- Houston KA, Henley SJ, Li J, White MC, Richards TB. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004-2009. Lung Cancer 2014; 86(1):22-8. doi: 10.1016/j.lungcan.2014.08.001 [Crossref] [ Google Scholar]
- Burns DM, Anderson CM, Gray N. Do changes in cigarette design influence the rise in adenocarcinoma of the lung?. Cancer Causes Control 2011; 22(1):13-22. doi: 10.1007/s10552-010-9660-0 [Crossref] [ Google Scholar]
- Mirtcheva RM, Vazquez M, Yankelevitz DF, Henschke CI. Bronchioloalveolar carcinoma and adenocarcinoma with bronchioloalveolar features presenting as ground-glass opacities on CT. Clin Imaging 2002; 26(2):95-100. doi: 10.1016/s0899-7071(01)00372-2 [Crossref] [ Google Scholar]
- Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 2014; 84(1):13-22. doi: 10.1016/j.lungcan.2014.01.009 [Crossref] [ Google Scholar]
- Thompson CA, Waldhör T, Schernhammer ES, Hackl M, Vutuc C, Haidinger G. Smoking and lung cancer: current trends in Austria. Wien Klin Wochenschr 2012; 124(15-16):493-9. doi: 10.1007/s00508-012-0207-0 [Crossref] [ Google Scholar]
- Nakamura H, Saji H. Worldwide trend of increasing primary adenocarcinoma of the lung. Surg Today 2014; 44(6):1004-12. doi: 10.1007/s00595-013-0636-z [Crossref] [ Google Scholar]
- Merchant TE, Kortmann RD. Pediatric Radiation Oncology. Cham: Springer; 2018.
- Matakidou A, Eisen T, Houlston RS. Systematic review of the relationship between family history and lung cancer risk. Br J Cancer 2005; 93(7):825-33. doi: 10.1038/sj.bjc.6602769 [Crossref] [ Google Scholar]
- Rezazadeh A, Laber DA, Ghim SJ, Jenson AB, Kloecker G. The role of human papilloma virus in lung cancer: a review of the evidence. Am J Med Sci 2009; 338(1):64-7. doi: 10.1097/MAJ.0b013e3181a393ba [Crossref] [ Google Scholar]
- Castro CY, Ostrowski ML, Barrios R, Green LK, Popper HH, Powell S. Relationship between Epstein-Barr virus and lymphoepithelioma-like carcinoma of the lung: a clinicopathologic study of 6 cases and review of the literature. Hum Pathol 2001; 32(8):863-72. doi: 10.1053/hupa.2001.26457 [Crossref] [ Google Scholar]
- Yu YH, Liao CC, Hsu WH, Chen HJ, Liao WC, Muo CH. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol 2011; 6(1):32-7. doi: 10.1097/JTO.0b013e3181fb4fcc [Crossref] [ Google Scholar]