Arch Iran Med. 27(3):168-173.
doi: 10.34172/aim.2024.25
Case Report
Synchronous Double Primary Angiosarcoma Originating from the Stomach and Rectum: A Case Report and a Literature Review
Tanju Kapagan Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, * 
Nilufer Bulut Conceptualization, Data curation, Methodology, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing, 1 
Gokmen Umut Erdem Conceptualization, Data curation, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing, 1 
Suleyman Yıldırım Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing, 2 
Zeynep Betul Erdem Investigation, Methodology, Resources, Supervision, Writing – review & editing, 3 
Halil Sahin Investigation, Methodology, Resources, Supervision, Writing – review & editing, 2 
Author information:
1Başakşehir Çam and Sakura City Hospital, Department of Medical Oncology, 34480 Istanbul, Turkey
2Başakşehir Çam and Sakura City Hospital, Department of Gastroenterology, 34480 Istanbul, Turkey
3Başakşehir Çam and Sakura City Hospital, Department of Pathology, 34480 Istanbul, Turkey
Abstract
Angiosarcomas originating from the gastrointestinal tract are rare but highly aggressive tumors with poor prognosis. These tumors can be misdiagnosed as benign and malignant gastrointestinal tract lesions. The definitive histological diagnosis of angiosarcomasis made by pathologists based on immunohistochemical analysis demonstrating cluster of differentiation 31 (CD31), factor VIII-related antigen (FVIIIRAg), erythroblast transformation specific related gene (ERG), and cluster of differentiation 34 (CD34). Angiosarcomas are treated with a single or multimodality approach that may include resection, radiotherapy, chemotherapy, and palliative care, depending on the stage of disease and the condition of the patient. No matter the treatment option, metastasis and death rates are substantially highin patients with angiosarcoma. In this context, a 59-year-old male with synchronous double primary angiosarcoma arising from the gastric and rectum who presented with the complaint of abdominal pain and distention to the outpatient clinic is presented in this case report, along with a brief literature review.
Keywords: Cancer, Gastrointestinal bleeding, Sarcoma, Synchronous angiosarcoma
Copyright and License Information
© 2024 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Kapagan T, Bulut N, Erdem GU, Yıldırım S, Erdem ZB, Sahin H. Synchronous double primary angiosarcoma originating from the stomach and rectum: a case report and a literature review. Arch Iran Med. 2024;27(3):168-173. doi: 10.34172/ aim.2024.25
Introduction
Angiosarcomas are aggressive tumors arising from blood and lymphatic vessels,1,2 often observed in men in the sixth and seventh decades.3-5 These tumors are likely to be confused with ulcerous lesions since they are rarely encountered in daily clinical practice, and thus cancer diagnosis is often missed.6 Angiosarcomas may be treated with a single or multi-modality approach that primarily involves chemotherapy, including anthracyclines, dacarbazine, cisplatin, vinca alkaloids, thalidomide agents, or tyrosine kinase inhibitors such as pazopanib, sorafenib, or sunitinib, along with surgical resection, radiotherapy, plasma argon coagulation, and palliative care.7,8 In light of the foregoing information, a 59-year-old male diagnosed with angiosarcoma both in the stomach and rectum with treatment unresponsiveness and short survival has been addressed in this case report.
Case Report
A 59-year-old male patient presented with a complaint of abdominal pain and distension. Abdominal ultrasonography revealed multiple cystic lesions in the hepatic and splenic parenchyma and free fluid in the abdomen. Follow-up magnetic resonance imaging demonstrated a massive amount of free fluid in the abdomen and multiple cystic lesions in the hepatic and splenic parenchyma, the largest of which measured 60 × 51 mm. The signal intensity of the lesions was hypointense on the T1-weighted image and hyperintense on the T2-weighted image. In addition, an increase in the symmetrical wall thickness was detected in the distal rectum. Upper endoscopy and colonoscopy examinations were requested for gastrointestinal (GI) tract cancer screening. Gastroscopy revealed a 1.5-cm ulcerated lesion that was surrounded by a raised hyperemic halo in the distal stomach corpus (Figure 1A), and colonoscopy represented a diminutive polyp in the rectum (Figure 1B).
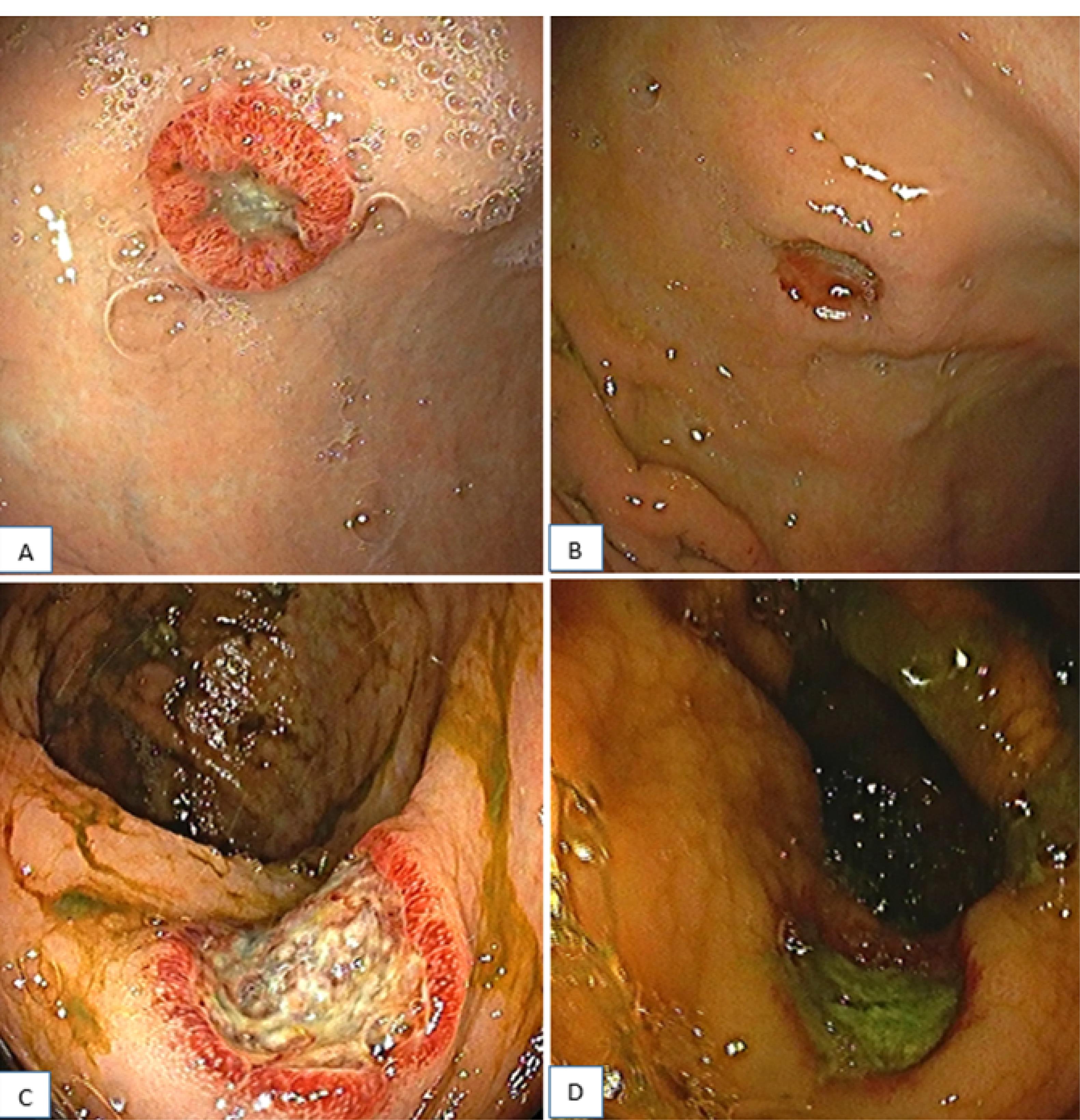
Figure 1.
The Endoscopic Findings of Angiosarcoma in the Stomach (A, B) and Rectum (C, D) Before (A, C) and After (B, D) Treatment
.
The Endoscopic Findings of Angiosarcoma in the Stomach (A, B) and Rectum (C, D) Before (A, C) and After (B, D) Treatment
Biopsy samples taken from the stomach and rectum had similar pathological features, suggesting endothelial angiosarcoma. Histopathologic examination of both localizations (i.e., stomach and rectum) revealed focally ulcerated and hyperplastic epithelium with a tumoral infiltration in the lamina propria. Neoplastic cells were spindled to be epithelioid-shaped with large, hyperchromatic, and pleomorphic nuclei, forming solid sheets, focally cleft-like spaces, and rarely intracytoplasmic lumens, expanding lamina propria around gastric and rectal crypts and glands. On immunohistochemical analyses, both tumors showed diffuse ERG (Figure 2 A-B), CD31 (Figure 2 C-D), and CD34 (Figure 2 E-F) expression, whereas cytokeratin [anti-cytokeratin monoclonal antibodies (AE1/AE3)], epithelial membrane antigen (EMA), human herpesvirus 8 (HHV8), special AT (adenine and thymine)-rich sequence-binding protein 2, sex-determining region Y-related high-mobility group box 10 protein (SOX10), 100% soluble protein (S100), and cytomegalovirus were all negative. Cytologic analysis of abdominal fluid and the fine needle aspiration biopsy taken from the cystic lesion demonstrated hemorrhagic effusion and mixed-type inflammatory cells in the hepatic region, respectively. The patient was started on 75 mg/m2 of doxorubicin once every 3 weeks. However, control gastroscopy and colonoscopy did not reveal any significant improvement in the gastric and rectal lesions after the completion of three cycles of treatment (Figure 1C-D). On the other hand, the positron emission tomography and computed tomography scans performed after the treatment revealed an increase in the 18F-fluorodeoxyglucose uptake in newly developed metastatic nodes in the supra/infra diaphragmatic regions. The patient’s general condition gradually deteriorated, and he died four months after diagnosis.
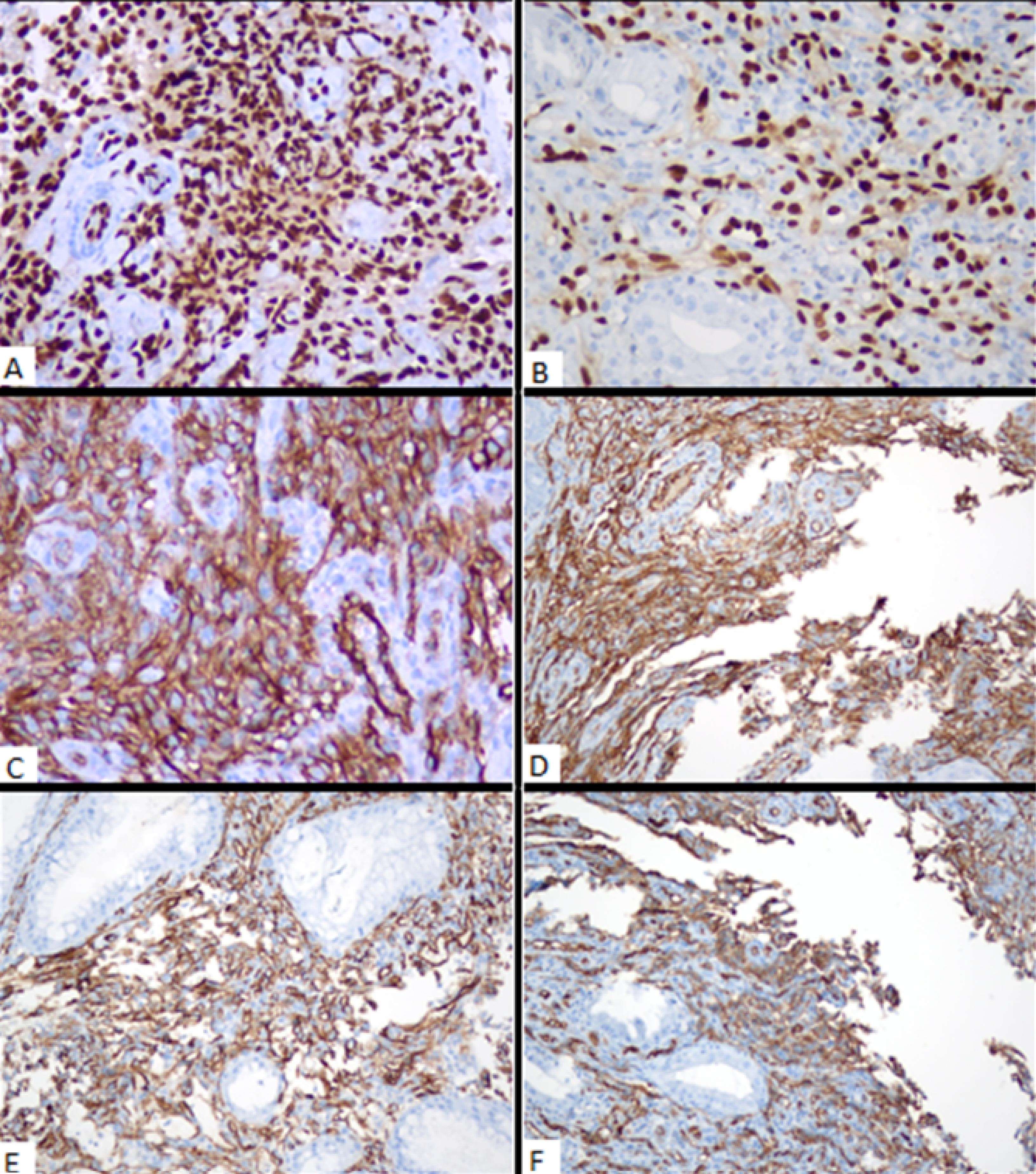
Figure 2.
ERG (A, B), CD31 (C, D), and CD34 (E, F) Immunohistochemical Findings of Angiosarcoma in the Stomach and Rectum, Respectively
.
ERG (A, B), CD31 (C, D), and CD34 (E, F) Immunohistochemical Findings of Angiosarcoma in the Stomach and Rectum, Respectively
Literature Review and Discussion
A thorough review of the literature revealed 23 cases diagnosed with synchronous double primary angiosarcoma originating from the GI system (Table 1).8-28
Table 1.
Cases of Synchronous Double Primary Angiosarcoma of the Gastrointestinal Tract (Modified From Zacarias Fohrding L et al
19)
|
Reference
|
Age (Years)/Gender
|
Initial Symptoms
|
Tumor Localizations
|
IHC
|
Radiation History
|
Treatment
|
Outcome
|
| Fukita et al9 |
80/M |
Melena and dyspnea |
Gastric, duodenum, and large bowel |
FVIIIRAg, CD31, and CD34 |
None |
None (PC) |
Died shortly |
| De Francesco et al10 |
68/M |
Haemoptysis and melena |
Gastric, small bowel, lung, and thyroid |
CD31 |
None |
Chemotherapy |
Died after 75 days |
| Navarro-Chagoya et al11 |
45/M |
Melena and weight loss |
Small bowel (multifocal) |
FVIIIRAg, CD31, and pancytokeratin |
Radiation |
Resection |
NA |
| Leong et al12 |
60/M |
Dyspnea and weakness |
Gastric and duodenum |
CD31, vimentin, and pancytokeratin |
NA |
Resection |
NA |
| Taxy and Battifora 13 |
64/M |
GI bleeding |
Small bowel (multifocal) |
FVIIIRAg and vimentin |
None |
Resection |
Died after one year |
| Taxy and Battifora 13 |
57/M |
NA |
Ileocecal valve, small bowel, and mesentery |
FVIIIRAg |
None |
Resection |
Died in several days |
| Louie et al14 |
78/M |
Failure to thrive |
Large bowel (multifocal) |
ERG, CD31 |
None |
None (PC) |
Died shortly |
| Brown et al15 |
77/M |
Rectal bleeding and obstructive bowel |
Rectum and sigmoid colon |
NA |
NA |
Resection |
Died after 6 months |
| Chen et al16 |
77/F |
Melena and dizziness |
Gastric and large bowel |
Pancytokeratin, CD31, CD34, EMA, and vimentin |
None |
Refused chemotherapy (PC) |
Died after 3 months |
| Radić et al17 |
61/M |
Fever (39°C), sweats, and vomiting |
Large bowel (multifocal) |
ERG, CD31, CD34, vimentin, and CD117 |
None |
Resection |
Died after 60 days |
| Pagni et al18 |
74/M |
Anemia and melena |
Gastric and recuml |
FVIIIRAg and CD31 |
NA |
None (PC) |
NA |
| Zacarias Föhrding et al19 |
84/M |
Anemia and melena |
Small bowel (multifocal) |
CD31, vimentin, and CD34 |
None |
Resection |
NA |
| Martínez-Alcalá García et al20 |
78/M |
Anemia |
Duodenum and jejunum |
CD31 |
None |
None (PC) |
Died after 4 months |
| Wolov et al21 |
80/F |
Peripheral edema and abdominal distension |
Small and large bowel |
FVIIIRAg |
Radiation |
Resection |
Died after 2 weeks |
| Wolov et al21 |
69/F |
Anorexia, weight loss, and hematochezia |
Small and large bowel |
FVIIIRAg |
Radiation |
Resection |
Died after 23 days |
| Shi et al22 |
62/M |
Appetite, weakness, melaena, and weight loss |
Jejunum (multifocal) |
Vimentin, CD31, CD34, and pancytokeratin |
None |
Resection |
NA |
| Policarpio-Nicolas et al23 |
51/F |
Appetite and abdominal pain |
Small bowel and appendix |
FVIIIRAg, CD31, and CD34 |
Radiation |
Resection |
Died after 10 months |
| Takahashi et al24 |
85/M |
Fever (40°C) and abdominal distension |
Small bowel (multifocal) |
FVIIIRAg, CD31, CD34, and vimentin |
None |
Resection |
Died after 42 days |
| Mohammed et al25 |
25/F |
Abdominal pain and weight loss |
Small and large bowel |
NA |
None |
Resection |
Died after 11 days |
| Delvaux et al26 |
67/M |
Weight loss, abdominal pain, and melena |
Small bowel (multifocal) |
FVIIIRAg, CD31, and CD34 |
None |
Resection |
Died after 3 months |
| Al Ali et al27 |
87/M |
Weakness |
Duodenum and jejunum |
NA |
None |
Endoscopy, argon plasma coagulation |
Died after 6 weeks |
| Grewal et al28 |
73/M |
Weakness and melena |
Duodenum and jejunum |
NA |
Radiation |
Resection |
Died after 4 months |
| Nai et al8 |
73/M |
Chest pain, dyspnea, and melena |
Duodenum and jejunum |
CD34, vimentin, Wilm’s tumor-1, and Vwf |
None |
Resection |
Died in a short time |
| Present case |
59/M |
Abdominal distention and pain |
Gastric and rectum |
ERG, CD31, and CD34 |
None |
Chemotherapy |
Died after 4 months |
Note. EMA: Epithelial membrane antigen; ERG: Erythroblast transformation specific related gene; FVIIIRAg: Factor VIII-related antigen; IHC: Immunohistochemical; NA: Not available; M: Male; F: Female; PC: Palliative care; Vwf: von Willebrand factor.
Demographic characteristics, initial symptoms, and immunohistochemical features of our case and these 23 cases, as well as the applied treatment approaches, were tabulated using descriptive statistics (Table 2).
Table 2.
Patients’ Age, Gender, Symptoms, Treatments, and Immunohistochemical Features
|
|
Age
|
|
Mean (y)
|
Median (y)
|
Minimum (y)-Maximum (y)
|
| Gender |
|
|
|
| Female (n = 5) |
60 |
69 |
25-80 |
| Male (n = 19) |
70 |
73 |
45-87 |
|
|
N
|
Percent
|
| Symptoms |
|
|
| GI bleeding |
13 |
54.20 |
| Weight loss |
5 |
20.80 |
| Weakness |
4 |
16.70 |
| Abdominal pain |
4 |
16.70 |
| Anemia |
3 |
12,.50 |
| Dyspnea |
3 |
12.50 |
| Abdominal distansiyon |
3 |
12.50 |
| Appetite |
2 |
8.30 |
| Fever |
2 |
8.30 |
| Anorexia |
1 |
4.20 |
| Chest pain |
1 |
4.20 |
| Dizziness |
1 |
4.20 |
| Failure to thrive |
1 |
4.20 |
| Obstructive bowel |
1 |
4.20 |
| Peripheral edema |
1 |
4.20 |
| Sweats vomiting |
1 |
4.20 |
| IHC markers |
|
|
| CD31 |
15 |
62.50 |
| FVIIIRAg |
10 |
41.60 |
| CD34 |
10 |
41.60 |
| Vimentin |
8 |
33.30 |
| Pancytokeratin |
4 |
16.70 |
| ERG |
3 |
12.50 |
| CD117 |
1 |
4.20 |
| CKAE1/AE3 |
1 |
4.20 |
| EMA |
1 |
4.20 |
| Vwf |
1 |
4.20 |
| Wilm’s tumor-1 |
1 |
4.20 |
| NA |
4 |
16.70 |
| Treatments |
|
|
| Resection |
16 |
66.7 |
| Palliative care |
5 |
20.8 |
| Chemotherapy |
2 |
8.3 |
| Endoscopic argon plasma coagulation |
1 |
4.2 |
Note. EMA: Epithelial membrane antigen; ERG: Erythroblast transformation specific related gene; FVIIIRAg: Factor VIII-related antigen; GI: Gastrointestinal; HC: Immunohistochemical; NA: Not available; M: Male; F: Female; vWF: von Willebrand factor.
In a study conducted by Schizas et al with 110 patients with angiosarcoma originating from the GI tract, of whom nearly 60% were male, whose mean age was approximately 62 years. In addition, 14 patients were diagnosed with synchronous double primary angiosarcoma originating from the GI system. The 6-month survival rate of these 14 patients, who mainly had symptoms such as GI bleeding, abdominal pain, and obstructive symptoms, was 23.08%. The univariate and multivariate logistic regression analyses revealed that surgical tumor resection was a significant factor in patients’ survival rates.29
In another study conducted on 25 angiosarcoma patients, a strong correlation was found between the presence of angiosarcoma and CD31 and ERG among the immunohistochemical parameters.30
In a systematic review, including 33 patients with primary colorectal angiosarcoma, it was concluded that the best treatment modality is either stand-alone complete surgical resection or complete surgical resection in combination with post-surgical chemotherapy.31
Another systematic review, including 12 patients with primary small bowel angiosarcoma, reported that 9 of the 10 patients who were treated with only one of the following treatment modalities of surgery, chemotherapy, or argon plasma coagulation died within 1 year.24
Conclusion
In conclusion, combination treatment with surgical resection remains a cornerstone treatment strategy for angiosarcomas. However, it is important to note that the rate of local recurrence, metastasis, or death as a result of diagnostic delays due to the aggressive nature of tumors is still extremely high regardless of the treatment options used alongside surgical resection.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Peng X, Duan Z, Yin H, Dai F, Liu H. Ovarian epithelioid angiosarcoma complicating pregnancy: a case report and review of the literature. J Int Med Res 2021; 49(5):3000605211019641. doi: 10.1177/03000605211019641 [Crossref] [ Google Scholar]
- Noujaim J, Thway K, Bajwa Z, Bajwa A, Maki RG, Jones RL. Epithelioid sarcoma: opportunities for biology-driven targeted therapy. Front Oncol 2015; 5:186. doi: 10.3389/fonc.2015.00186 [Crossref] [ Google Scholar]
- Bhaludin BN, Thway K, Adejolu M, Renn A, Kelly-Morland C, Fisher C. Imaging features of primary sites and metastatic patterns of angiosarcoma. Insights Imaging 2021; 12(1):189. doi: 10.1186/s13244-021-01129-9 [Crossref] [ Google Scholar]
- Spiker AM, Mangla A, Ramsey ML. Angiosarcoma. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022.
- Lydiatt WM, Shaha AR, Shah JP. Angiosarcoma of the head and neck. Am J Surg 1994; 168(5):451-4. doi: 10.1016/s0002-9610(05)80097-2 [Crossref] [ Google Scholar]
- Sharko A, Samuel S, Ying GW, Prasad S, Baig S. Gastric epithelioid angiosarcoma: an unexpected tumor in an unexpected location. Cureus 2021; 13(5):e15049. doi: 10.7759/cureus.15049 [Crossref] [ Google Scholar]
- Grünwald V, Karch A, Schuler M, Schöffski P, Kopp HG, Bauer S. Randomized comparison of pazopanib and doxorubicin as first-line treatment in patients with metastatic soft tissue sarcoma age 60 years or older: results of a German intergroup study. J Clin Oncol 2020; 38(30):3555-64. doi: 10.1200/jco.20.00714 [Crossref] [ Google Scholar]
- Nai Q, Ansari M, Liu J, Razjouyan H, Pak S, Tian Y. Primary small intestinal angiosarcoma: epidemiology, diagnosis and treatment. J Clin Med Res 2018; 10(4):294-301. doi: 10.14740/jocmr3153w [Crossref] [ Google Scholar]
- Fukita Y, Yasuda I, Ishibashi H, Asaki T, Adachi S, Toyomizu M. [Multifocal angiosarcoma of the gastrointestinal tract: a case report]. Nihon Shokakibyo Gakkai Zasshi 2017; 114(9):1665-74. doi: 10.11405/nisshoshi.114.1665.[Japanese] [Crossref] [ Google Scholar]
- De Francesco V, Bellesia A, Corsi F, Pennella A, Ridola L, Zullo A. Multifocal gastrointestinal angiosarcoma: a challenging diagnosis?. J Gastrointestin Liver Dis 2015; 24(4):519-22. doi: 10.15403/jgld.2014.1121.244.asm [Crossref] [ Google Scholar]
- Navarro-Chagoya D, Figueroa-Ruiz M, López-Gómez J, Nava-Leyva H, Álvarez-Ponce CE, Guzmán-Sombrero G. Obscure gastrointestinal bleeding due to multifocal intestinal angiosarcoma. Int J Surg Case Rep 2015; 10:169-72. doi: 10.1016/j.ijscr.2015.03.049 [Crossref] [ Google Scholar]
- Leong J, Rascon MA, Kaushik N. Multifocal angiosarcoma of the gastrointestinal tract. Endoscopy 2008; 40 Suppl 2:E252-3. doi: 10.1055/s-2008-1077645 [Crossref] [ Google Scholar]
- Taxy JB, Battifora H. Angiosarcoma of the gastrointestinal tract A report of three cases. Cancer 1988; 62(1):210-6. doi: 10.1002/1097-0142(19880701)62:1<210::aidcncr2820620132>3.0.co;2-8 [Crossref] [ Google Scholar]
- Louie J, Tejaswi S, Matsukuma K. Colonic angiosarcoma: a rare gastrointestinal malignancy. Clin Gastroenterol Hepatol 2020; 18(7):e75. doi: 10.1016/j.cgh.2019.04.019 [Crossref] [ Google Scholar]
- Brown CJ, Falck VG, MacLean A. Angiosarcoma of the colon and rectum: report of a case and review of the literature. Dis Colon Rectum 2004; 47(12):2202-7. doi: 10.1007/s10350-004-0698-5 [Crossref] [ Google Scholar]
- Chen YW, Dong J, Chen WY, Dai YN. Multifocal gastrointestinal epithelioid angiosarcomas diagnosed by endoscopic mucosal resection: a case report. World J Gastroenterol 2020; 26(29):4372-7. doi: 10.3748/wjg.v26.i29.4372 [Crossref] [ Google Scholar]
- Radić S, Zovak M, Galović Marić A, Baturina S, Kirigin MS, Krušlin B. Multiple primary angiosarcomas of the colon. Case Rep Pathol 2021; 2021:7237379. doi: 10.1155/2021/7237379 [Crossref] [ Google Scholar]
- Pagni F, Leone BE, Ronchi S, Sartori P, Corti L, Maggioni D. Multicentric CD34 negative epithelioid angiosarcoma of the digestive system. Acta Gastroenterol Belg 2012; 75(4):466. [ Google Scholar]
- Zacarias Föhrding L, Macher A, Braunstein S, Knoefel WT, Topp SA. Small intestine bleeding due to multifocal angiosarcoma. World J Gastroenterol 2012; 18(44):6494-500. doi: 10.3748/wjg.v18.i44.6494 [Crossref] [ Google Scholar]
- Martínez-Alcalá García F, Pérez Pozo JM, Ciria Ávila JA, Galera Davidson H, Martínez Alcalá F. [Primary multifocal epitheliod angiosarcoma as an exceptional cause of chronic anemia due to blood loss in the digestive tract]. Gastroenterol Hepatol 2012; 35(4):295-6. doi: 10.1016/j.gastrohep.2011.11.006.[Spanish] [Crossref] [ Google Scholar]
- Wolov RB, Sato N, Azumi N, Lack EE. Intra-abdominal “angiosarcomatosis” report of two cases after pelvic irradiation. Cancer 1991; 67(9):2275-9. doi: 10.1002/1097-0142(19910501)67:9<2275::aidcncr2820670911>3.0.co;2-6 [Crossref] [ Google Scholar]
- Shi H, Zhen T, Zhang F, Dong Y, Han A. Multifocal epithelioid angiosarcoma of the jejunum: a case report. Pathology 2016; 48(1):91-3. doi: 10.1016/j.pathol.2015.11.013 [Crossref] [ Google Scholar]
- Policarpio-Nicolas ML, Nicolas MM, Keh P, Laskin WB. Postradiation angiosarcoma of the small intestine: a case report and review of literature. Ann Diagn Pathol 2006; 10(5):301-5. doi: 10.1016/j.anndiagpath.2005.09.006 [Crossref] [ Google Scholar]
- Takahashi M, Ohara M, Kimura N, Domen H, Yamabuki T, Komuro K. Giant primary angiosarcoma of the small intestine showing severe sepsis. World J Gastroenterol 2014; 20(43):16359-63. doi: 10.3748/wjg.v20.i43.16359 [Crossref] [ Google Scholar]
- Mohammed A, Aliyu HO, Liman AA, Abdullahi K, Abubakar N. Angiosarcoma of the small intestine. Ann Afr Med 2011; 10(3):246-8. doi: 10.4103/1596-3519.84702 [Crossref] [ Google Scholar]
- Delvaux V, Sciot R, Neuville B, Moerman P, Peeters M, Filez L. Multifocal epithelioid angiosarcoma of the small intestine. Virchows Arch 2000; 437(1):90-4. doi: 10.1007/s004280000183 [Crossref] [ Google Scholar]
- Al Ali J, Ko HH, Owen D, Steinbrecher UP. Epithelioid angiosarcoma of the small bowel. Gastrointest Endosc 2006; 64(6):1018-21. doi: 10.1016/j.gie.2006.04.020 [Crossref] [ Google Scholar]
- Grewal JS, Daniel AR, Carson EJ, Catanzaro AT, Shehab TM, Tworek JA. Rapidly progressive metastatic multicentric epithelioid angiosarcoma of the small bowel: a case report and a review of literature. Int J Colorectal Dis 2008; 23(8):745-56. doi: 10.1007/s00384-007-0420-x [Crossref] [ Google Scholar]
- Schizas D, Mastoraki A, Giannakodimos I, Giannakodimos A, Ziogou A, Katsaros I. Primary angiosarcoma of the gastrointestinal tract: a systematic review of the literature. J Invest Surg 2022; 35(2):400-8. doi: 10.1080/08941939.2020.1853283 [Crossref] [ Google Scholar]
- Sullivan HC, Edgar MA, Cohen C, Kovach CK, HooKim K, Reid MD. The utility of ERG, CD31 and CD34 in the cytological diagnosis of angiosarcoma: an analysis of 25 cases. J Clin Pathol 2015; 68(1):44-50. doi: 10.1136/jclinpath-2014-202629 [Crossref] [ Google Scholar]
- Wang Q, Zhao T, Mi BT, Zhang YL, Wei R, Tong HL. Primary colonic angiosarcoma seen in a patient on calcium channel blocker: a case report with summary analysis of 32 other cases from the literature. Am J Case Rep 2018; 19:254-61. doi: 10.12659/ajcr.907287 [Crossref] [ Google Scholar]