Arch Iran Med. 28(2):124-128.
doi: 10.34172/aim.28751
Case Report
Lactating Adenoma in Pregnancy: Report of Three Cases
Julija Cvetković Conceptualization, Visualization, Writing – original draft, 1, * 
Maja Jovičić Milentijević Supervision, Visualization, Writing – review & editing, 1
Nikola Živković Conceptualization, Methodology, Validation, Writing – original draft, 1
Miodrag Đorđević Investigation, Resources, 2
Jelena Grujović Investigation, Resources, 1
Goran Radenković Funding acquisition, Methodology, Supervision, Writing – review & editing, 3
Author information:
1Center for Pathology, University Clinical Center Niš, 18000 Niš, Serbia
2Clinic for Endocrine Surgery, University Clinical Center Niš, 18000 Niš, Serbia
3Department of Histology and Embryology, Faculty of Medicine, University of Niš, 18000 Niš, Serbia
Abstract
Background:
Lactating adenoma is an infrequent benign stromal breast tumor mostly seen during pregnancy and lactation. This report seeks to enhance the literature by presenting three cases diagnosed with this condition, while also highlighting its histological subtypes, immunohistochemical characteristics, and differential diagnoses.
Case Presentation:
In all three patients, a circumscribed, painless, and mobile mass was found in the breast in the third trimester of pregnancy. Pathohistological examination revealed hyperplastic lobules with glandular formations in back-to-back arrangements showing more or less abundant hobnailing phenomena with intraluminal eosinophilic secretions and inconspicuous myoepithelial cell layer separated by fibrovascular stroma. The immune profile showed positive reaction for cytokeratin 14 and p40 markers, indicating the presence of myoepithelial cells and distinguishing these cases from other breast lesions.
Conclusion:
Pathohistological and immunohistochemical analysis should be performed to differentiate these lesions from other benign lesions and malignant tumors.
Keywords: Adenoma, Breast, Case reports, Lactation, Pregnancy
Copyright and License Information
© 2025 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Cvetković J, Jovičić Milentijević M, Živković N, Đorđević M, Grujović J, Radenković G. Lactating adenoma in pregnancy: report of three cases. Arch Iran Med. 2025;28(2):124-128. doi: 10.34172/aim.28751
Introduction
Lactating adenoma is a benign tumor of the breast that is mostly seen during pregnancy and lactation.1 Most commonly, they affect primigravidae between 20 and 35 years of age in the last trimester of pregnancy or lactation.2,3 Lactating adenomas are usually small in size (about 3 cm), painless, solid, well-circumscribed, and mobile nodule masses that tend to grow slowly.4-7 Pathophysiologically, it has not been fully investigated whether these adenomas arise de novo or in already existing hyperplastic lesions.
Some theories may refer to pre-existing fibroadenoma, tubular, or lobular adenoma under hormonal changes.2 However, lack of mediator complex subunit (MED) 12 exon 2 mutations, commonly seen in fibroadenomas, weakens this hypothesis.8 Others suggest that they develop de novo due to heightened levels of estrogen. On the other hand, pathohistological examination and differentiation of lactating adenoma from fibroadenoma could be challenging.9 Lactating adenomas are distinct from fibroadenomas, as they primarily consist of epithelial elements with minimal stromal tissue and lack the myoepithelial cell layer.10 Clinically, lactating adenomas present similarly to fibroadenomas, with a note that they typically regress after cessation of breastfeeding and can occur in succeeding pregnancies.11,12 Generally, these masses represent a diagnostic challenge because elevated hormone levels during pregnancy promote neoangiogenesis and glandular tissue proliferation, while on the other hand, the stromal component is reduced.10,13 Ultrasound features suggestive of lactating adenomas include a spherical hypo/isoechoic lesion with posterior enhancement and sharp edges, which may sometimes contain infarcted cystic fields.14 Although lactating adenomas are generally considered benign, there have been rare instances of their coexistence with invasive carcinomas.15-17 The golden standard in diagnosing lactating adenomas is a biopsy followed by pathohistological analysis.18 These cases emphasize the need for thorough evaluation of breast nodules during pregnancy, as the incidence of breast cancer in pregnant patients is increasing and timely diagnosis is crucial for effective patient management, which should not be postponed until after childbirth.
Case Report
Here, we present three cases of females with lactating adenomas. All three patients had a similar clinical presentation, which included a change in their breasts in the third trimester of pregnancy. A circumscribed, painless, and mobile mass was discovered in all three women. There was no change in the skin, such as depression, discoloration, or dimpling. No axillary lymphadenopathy was detected. They were not on any medications and had no allergies. A history of smoking was denied, and they drank alcohol occasionally. Ultrasonographically, these neoplasms appeared in two patients as spherical masses with parallel orientation, well-defined edges, iso-to-hypoechoic echo pattern, and posterior enhancement. However, an irregular, echogenic, vascularized change in the lactiform canal was observed on ultrasound of the third patient. All three patients had a score of 3/4 in Breast Imaging-Reporting and Data System (BIRADS). Surgical removal of the changes was indicated, followed by pathohistological analysis. The pregnancies of all three patients ended favorably, with the birth of three healthy children. The patients showed no recurrence or complications during the one-month postoperative follow-up.
A gross examination of the specimen from patient 1 revealed a fragment of fatty and glandular tissue, white in color, measuring 55 × 53 × 15 mm. On the cross-section, there was adipose tissue interspersed with bands of glandular tissue, with hemorrhage in the surrounding area. Microscopic examination revealed a well-defined proliferation of hyperplastic lobules that were densely packed, with layers of epithelial and myoepithelial cells divided by thin, delicate stromal tissue. The cuboidal and some hobnail-shaped cells lined the glandular formations with abundant intraluminal eosinophilic secretions. These cells lacked cytological atypia, their nuclei were small and round, and they had granular to vacuolated cytoplasm. Other micromorphological findings included typical lobular hyperplasia on the resection margins of the specimen.
The surgical specimen from patient 2 grossly consisted of two tissue fragments representing the fatty and glandular white to greyish breast tissue, measuring 30 mm and 35 mm. Microscopically, the preparation showed greatly expanded lobules of variably sized glands in a back-to-back arrangement. The hyperplastic lobules were separated by delicate fibrovascular septae. The closely packed glands comprised prominent nuclear hobnailing with bulbous nuclear projections into the lumen and an inconspicuous myoepithelial cell layer (Figure 1). However, the eosinophilic secretion in the lumen of the glands was scant. The hobnail-shaped cells were not atypical and had no pathological mitotic figures. Their cytoplasm was granular and vacuolated, and the nuclei were small and round with variably prominent small, pinpoint nucleoli. Other pathological findings of the specimen included chronic periductal and intralobular mastitis with focal exacerbation.
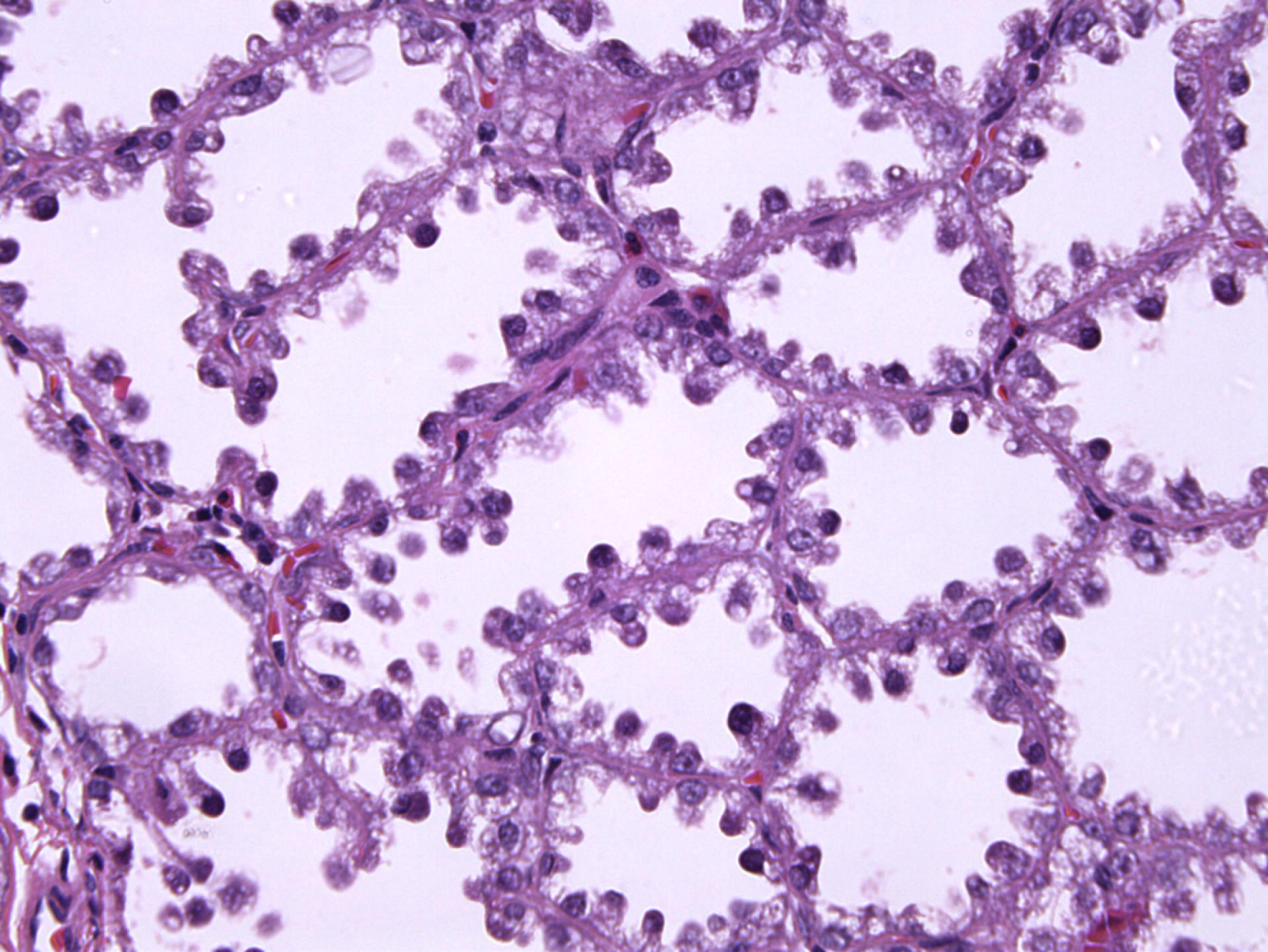
Figure 1.
Prominent Nuclear Hobnailing with Bulbous Nuclear Projections into the Lumen and an Inconspicuous Myoepithelial Cell Layer (Patient 2) (H&E × 400)
.
Prominent Nuclear Hobnailing with Bulbous Nuclear Projections into the Lumen and an Inconspicuous Myoepithelial Cell Layer (Patient 2) (H&E × 400)
Gross examination of the specimen from patient 3 revealed two tissue samples of greyish-white color measuring 109 mm and 5 mm. Micromorphological examination of the preparation revealed well-circumscribed lobular hyperplasia with slightly thicker strands of intervening stroma. The glands consisted of cuboidal and myoepithelial cells in mostly tubular histological patterns, with a smaller percentage of cribriform and solid patterns. Glandular cells were oval to round, showing mild cytological atypia with rare mitotic activity. Their cytoplasm was basophilic to granular with small, round or elongated nuclei and prominent nucleoli. Hobnail-shaped cells and secretion into the lumen were scarce (Figure 2). In the surrounding adipose and fibrous tissue, nonspecific chronic inflammatory infiltrate was found.
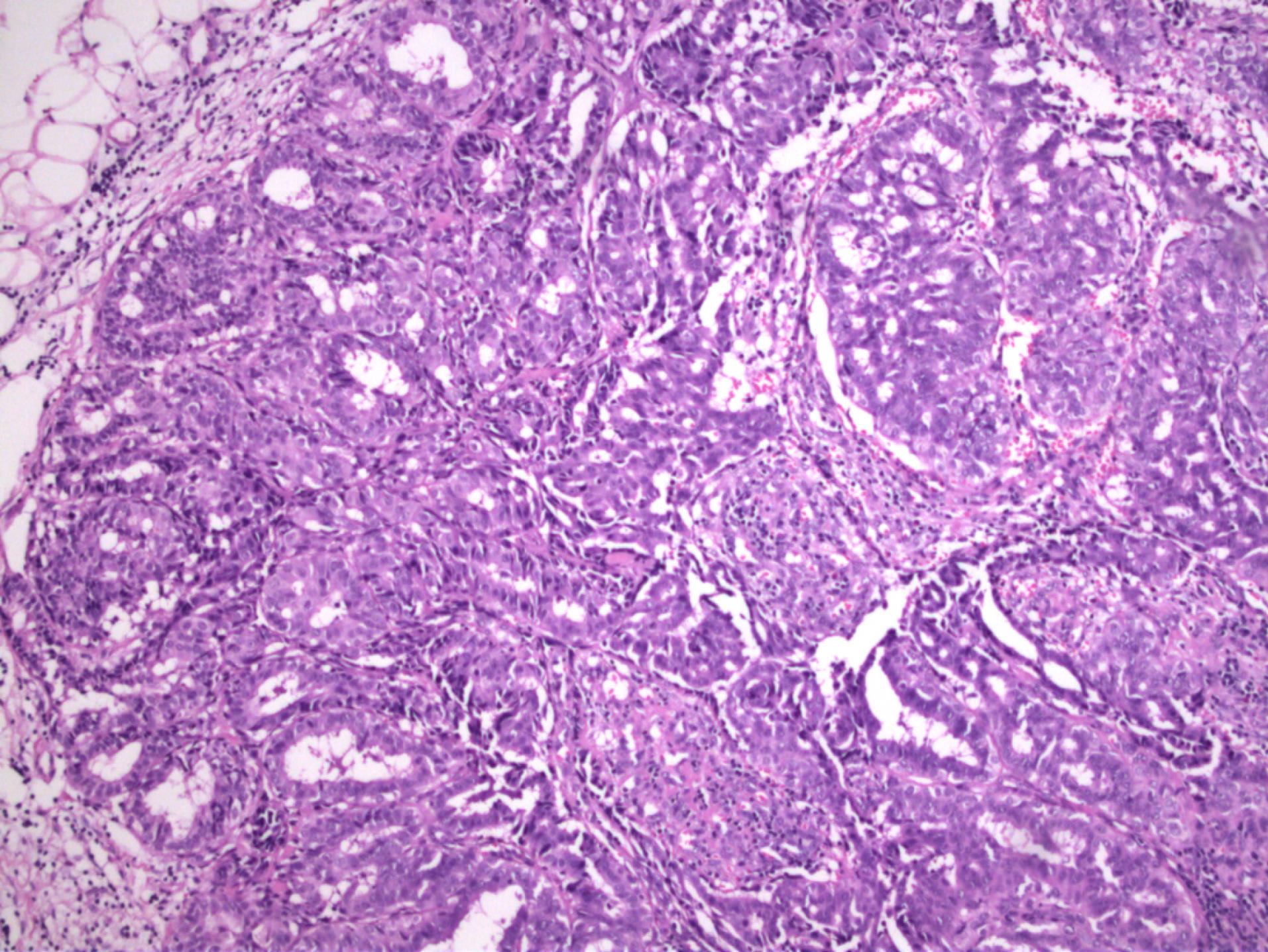
Figure 2.
Lobules Separated by Slightly Thicker Strands of Intervening Stroma. Glands consisted of cuboidal and myoepithelial cells in mostly tubular histological patterns, with a smaller percentage of cribriform and solid patterns. glandular cells were oval to round, showing mild cytological atypia with rare mitotic activity. Cytoplasm was basophilic to granular with small, round, or elongated nuclei and prominent nucleoli. Hobnail-shaped cells and secretion into the lumen were scarce (patient 3) (H&E × 100)
.
Lobules Separated by Slightly Thicker Strands of Intervening Stroma. Glands consisted of cuboidal and myoepithelial cells in mostly tubular histological patterns, with a smaller percentage of cribriform and solid patterns. glandular cells were oval to round, showing mild cytological atypia with rare mitotic activity. Cytoplasm was basophilic to granular with small, round, or elongated nuclei and prominent nucleoli. Hobnail-shaped cells and secretion into the lumen were scarce (patient 3) (H&E × 100)
In order to establish a definitive diagnosis and differentiate lactating adenoma from other breast diseases, an immunohistochemical analysis was performed. The immune profile showed a positive reaction for cytokeratin (CK) 14 and p40 markers, which indicated the presence of myoepithelial cells and distinguished these cases from other breast lesions (Figures 3 and 4).
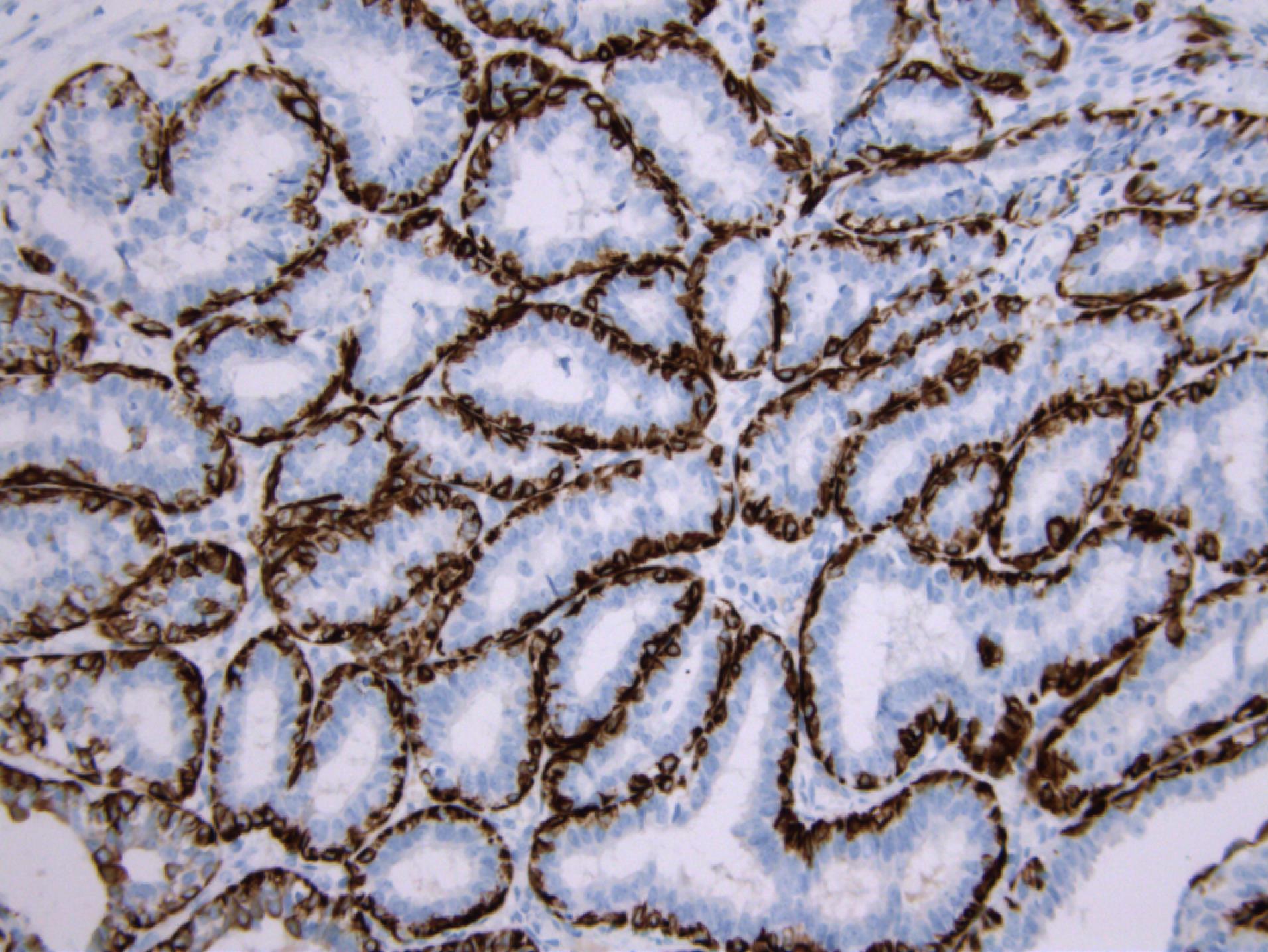
Figure 3.
CK14 Immunoreactivity Present in the Myoepithelial Cell Layer of the Glandular Formations (CK14 immunohistochemistry × 200)
.
CK14 Immunoreactivity Present in the Myoepithelial Cell Layer of the Glandular Formations (CK14 immunohistochemistry × 200)
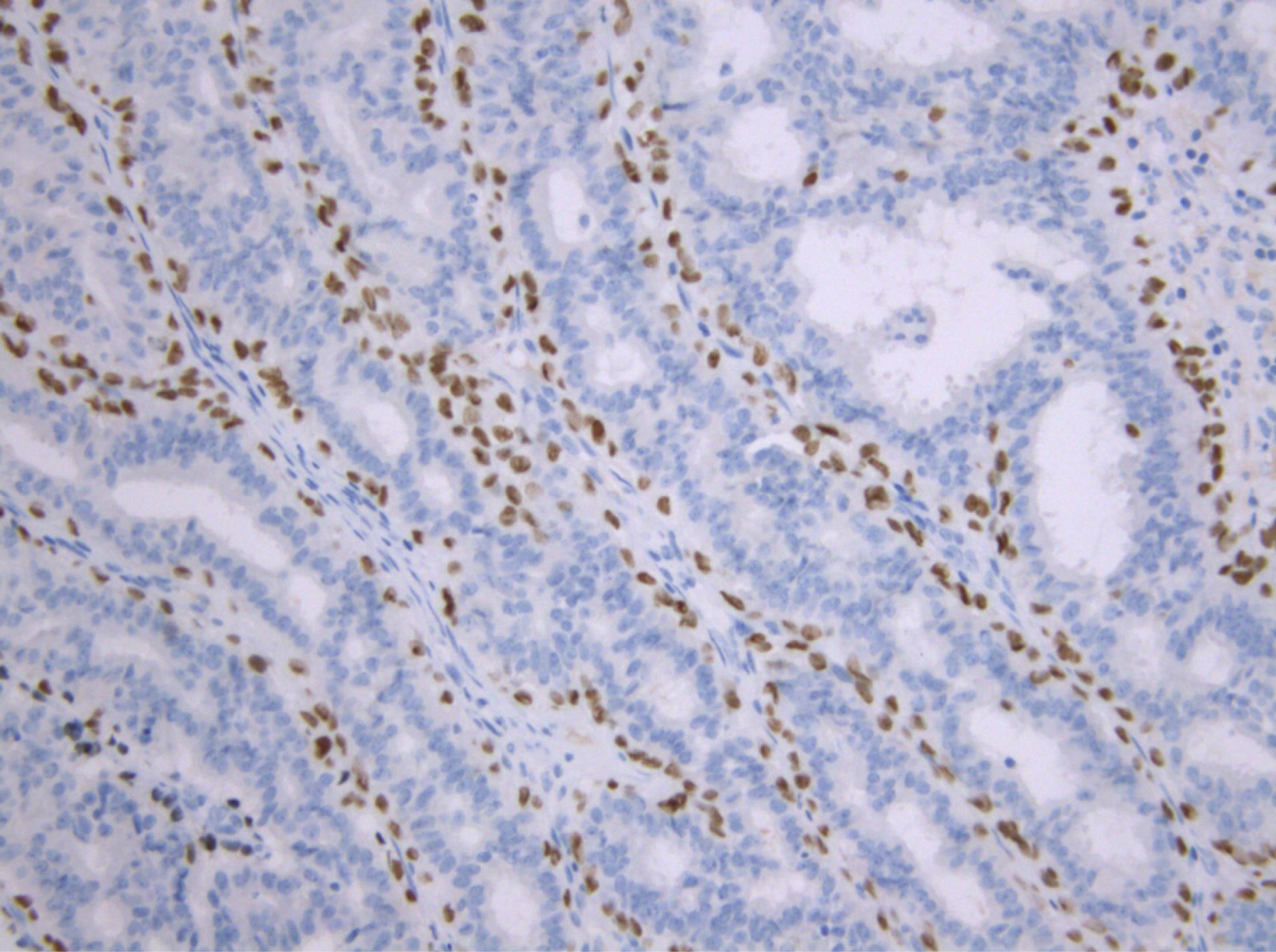
Figure 4.
p40 Immunostain Positive in the Myoepithelial Cell Layer of the Glandular Formations (p40 immunochemistry × 200).
.
p40 Immunostain Positive in the Myoepithelial Cell Layer of the Glandular Formations (p40 immunochemistry × 200).
Discussion
During pregnancy, hormones can cause various changes in the breasts, including the appearance of palpable masses, which further complicates both physical and radiological examination.19 Most lumps in pregnancy and lactation include lactating adenomas, fibroadenomas, and galactoceles.20,21 Given the fact that 3% of biopsied breast tissue samples during pregnancy and lactation are malignant tumors, any rapidly growing solid breast mass should be biopsied.22,23 On ultrasound, malignant changes can sometimes be masked by benign changes, especially if the mass has irregular borders, as in one of our patients. Changes of this type are usually well-defined solid, oval and lobular masses, around 3 cm in diameter.24 A homogeneous, hypoechoic appearance followed by posterior enhancement constitute the main characteristics of these lesions.25 Although most lactating adenomas tend to involute on their own, diagnosing them can be complex and surgical therapy may be necessary to rule out malignancy, particularly if they grow in size.26
Pathohistologically, they consist of a well-defined proliferation of densely packed hyperplastic lobules, featuring layers of epithelial and myoepithelial cells segregated by scanty stromal tissue. Cells lining the glands are cuboidal or hobnail-shaped, they possess small round nuclei and clear vacuolated cytoplasm. Normally, the cells do not show signs of cytological atypia and can bear a resemblance to pregnancy-like changes.27,28 Despite this, histological analysis of one patient’s specimen showed signs of mild atypia, rare mitotic figures, and a smaller number of hobnail-shaped cells with intraluminal secretion. Lactational changes, in addition to those seen in lactating adenomas, can also appear focally in other breast lesions that are not associated with pregnancy, such as fibroadenomas.29,30 When the histological presentation is characteristic, discerning a lactating adenoma from a fibroadenoma usually does not present any difficulties. Fibroadenomas that develop extensive lactational change may exhibit converging characteristics with lactating adenomas. Fibroadenomas represent benign biphasic neoplasms composed of both glandular and stromal components of terminal duct lobular unit, while lactating adenomas primarily consist of epithelial component with conspicuous lactational changes. Generally, the presence of the stromal component can be enough to make a difference between a lactating adenoma and a fibroadenoma with lactational changes.31
Furthermore, some malignant neoplasms can pose a greater challenge in histological differentiation. The main distinctive mark of secretory carcinoma is the presence of vacuolated, foamy cytoplasm of tumor cells and abundant intracellular and extracellular mostly eosinophilic secretions. Distinction between these two entities may be hindered when the secretory carcinoma is well differentiated, with only mild cytological atypia and indicative secretion.32 It is essential to review the patient’s history and medical background in such ambiguous situations. Secretory carcinoma primarily affects younger individuals and is not linked to pregnancy.33
Conclusion
Lactating adenoma requires a comprehensive evaluation to rule out malignancy, including physical examination, imaging, and histological findings. A thorough, individual approach is essential for every patient experiencing changes in the breast during pregnancy or lactation. This is crucial because delayed detection of malignancies in these specific conditions can lead to a worse prognosis and lower survival rates for women. Surgical therapy should be always performed when there is a family history of breast cancer, previous breast malignancy, or unclear and atypical radiological findings. Regarding the pathohistological evaluation of such cases, one should be careful and thorough to avoid overdiagnosing and declaring a benign condition malignant. Because of the specificity of these cases and doubtful micromorphological findings, immunohistochemistry needs to be performed to distinguish and confirm the benign nature of the condition.
Competing Interests
The authors declare that they have no competing interests.
Data Availability Statement
Data is available on request from the corresponding author.
Ethical Approval
Ethics approval and consent to participate: The study was approved by the by the Ethics Committee of the University Clinical Center Nis under number 34794/3. Written informed consent was taken from the patients. The identity of the patients is not revealed in the pictures or in the manuscript.
Funding
This study was funded by the internal project of the Faculty of Medicine, University of Niš (no. 38/20).
References
- Baker TP, Lenert JT, Parker J, Kemp B, Kushwaha A, Evans G. Lactating adenoma: a diagnosis of exclusion. Breast J 2001; 7(5):354-7. doi: 10.1046/j.1524-4741.2001.20075.x [Crossref] [ Google Scholar]
- Hertel BF, Zaloudek C, Kempson RL. Breast adenomas. Cancer 1976; 37(6):2891-905. doi: 10.1002/1097-0142(197606)37:6<2891::aid-cncr2820370647>3.0.co;2-p [Crossref] [ Google Scholar]
- James K, Bridger J, Anthony PP. Breast tumour of pregnancy (‘lactating’ adenoma). J Pathol 1988; 156(1):37-44. doi: 10.1002/path.1711560109 [Crossref] [ Google Scholar]
- Teng CY, Diego EJ. Case report of a large lactating adenoma with rapid antepartum enlargement. Int J Surg Case Rep 2016; 20:127-9. doi: 10.1016/j.ijscr.2016.01.027 [Crossref] [ Google Scholar]
- Kumar H, Narasimha A, Bhaskaran Bhaskaran, Divya Rani MN. Concurrent lactating adenoma and infiltrating ductal carcinoma: a case report. J Clin Diagn Res 2015; 9(8):ED14-5. doi: 10.7860/jcdr/2015/12786.6326 [Crossref] [ Google Scholar]
- Elzahaby IA, Saleh S, Metwally IH, Fathi A, Atallah K. Huge lactating adenoma of the breast: Case report. Breast Dis 2017; 37(1):37-42. doi: 10.3233/bd-160263 [Crossref] [ Google Scholar]
- Barco Nebreda I, Vidal MC, Fraile M, Canales L, González C, Giménez N. Lactating adenoma of the breast. J Hum Lact 2016; 32(3):559-62. doi: 10.1177/0890334416646564 [Crossref] [ Google Scholar]
- Volckmar AL, Leichsenring J, Flechtenmacher C, Pfarr N, Siebolts U, Kirchner M. Tubular, lactating, and ductal adenomas are devoid of MED12 Exon2 mutations, and ductal adenomas show recurrent mutations in GNAS and the PI3K-AKT pathway. Genes Chromosomes Cancer 2017; 56(1):11-7. doi: 10.1002/gcc.22396 [Crossref] [ Google Scholar]
- Sabate JM, Clotet M, Torrubia S, Gomez A, Guerrero R, de las Heras P. Radiologic evaluation of breast disorders related to pregnancy and lactation. Radiographics 2007; 27 Suppl 1:S101-24. doi: 10.1148/rg.27si075505 [Crossref] [ Google Scholar]
- Harris JR, Lippman ME, Morrow M, Osborne CK. Diseases of the Breast. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014.
- O’Hara MF, Page DL. Adenomas of the breast and ectopic breast under lactational influences. Hum Pathol 1985; 16(7):707-12. doi: 10.1016/s0046-8177(85)80156-8 [Crossref] [ Google Scholar]
- Hara Y, Yano H, Yamaguchi R, Iwasaki K. Surgical excision of a lactating adenoma with rapid enlargement: a case report. Int J Surg Case Rep 2021; 89:106544. doi: 10.1016/j.ijscr.2021.106544 [Crossref] [ Google Scholar]
- Kulkarni D. Clinical presentations of breast disorders in pregnancy and lactation. In: Alipour S, Omranipour R, eds. Diseases of the Breast during Pregnancy and Lactation. Cham: Springer; 2020. p. 33-9. 10.1007/978-3-030-41596-9_5.
- Parker S, Saettele M, Morgan M, Stein M, Winkler N. Spectrum of pregnancy- and lactation-related benign breast findings. Curr Probl Diagn Radiol 2017; 46(6):432-40. doi: 10.1067/j.cpradiol.2016.12.013 [Crossref] [ Google Scholar]
- Saglam A, Can B. Coexistence of lactating adenoma and invasive ductal adenocarcinoma of the breast in a pregnant woman. J Clin Pathol 2005; 58(1):87-9. doi: 10.1136/jcp.2004.018275 [Crossref] [ Google Scholar]
- Geschickter CF, Lewis D. Pregnancy and lactation changes in fibro-adenoma of breast. Br Med J 1938;1(4026):499-526.2. 10.1136/bmj.1.4026.499.
- Langer A, Mohallem M, Berment H, Ferreira F, Gog A, Khalifa D. Breast lumps in pregnant women. Diagn Interv Imaging 2015; 96(10):1077-87. doi: 10.1016/j.diii.2015.07.005 [Crossref] [ Google Scholar]
- Szabo J, Garcia D, Ciomek N, Margolies L. Spuriously aggressive features of a lactating adenoma prompting repeated biopsies. Radiol Case Rep 2017; 12(2):215-8. doi: 10.1016/j.radcr.2017.01.019 [Crossref] [ Google Scholar]
- Maia AF, Solinho M, Vicente Costa R, Alves O, Nogueira M. The importance of carefully evaluating breast masses during pregnancy. Acta Med Port 2023; 36(2):143-4. doi: 10.20344/amp.19118 [Crossref] [ Google Scholar]
- Yu JH, Kim MJ, Cho H, Liu HJ, Han SJ, Ahn TG. Breast diseases during pregnancy and lactation. Obstet Gynecol Sci 2013; 56(3):143-59. doi: 10.5468/ogs.2013.56.3.143 [Crossref] [ Google Scholar]
- Collins JC, Liao S, Wile AG. Surgical management of breast masses in pregnant women. J Reprod Med 1995; 40(11):785-8. [ Google Scholar]
- Monib S, Elkorety M. Giant lactating adenoma - size of a shot put ball. Eur J Case Rep Intern Med 2020; 7(5):001579. doi: 10.12890/2020_001579 [Crossref] [ Google Scholar]
- Son EJ, Oh KK, Kim EK. Pregnancy-associated breast disease: radiologic features and diagnostic dilemmas. Yonsei Med J 2006; 47(1):34-42. doi: 10.3349/ymj.2006.47.1.34 [Crossref] [ Google Scholar]
- Darling ML, Smith DN, Rhei E, Denison CM, Lester SC, Meyer JE. Lactating adenoma: sonographic features. Breast J 2000; 6(4):252-6. doi: 10.1046/j.1524-4741.2000.99093.x [Crossref] [ Google Scholar]
- Trengginas SS, Soewoto W. Lactating adenoma in pregnancy: a case report. Bioscientia Medicina: Journal of Biomedicine and Translational Research 2022; 6(8):2090-6. doi: 10.37275/bsm.v6i8.560 [Crossref] [ Google Scholar]
- Wakama IE, Fubara BN, Eli S, Okagua KE, Ocheche U. Lactating adenoma: a case report. Int J Innov Sci Res Technol 2023; 8(2):2254-6. doi: 10.5281/zenodo.7771035 [Crossref] [ Google Scholar]
- Langer A, Mohallem M, Berment H, Ferreira F, Gog A, Khalifa D. Breast lumps in pregnant women. Diagn Interv Imaging 2015; 96(10):1077-87. doi: 10.1016/j.diii.2015.07.005 [Crossref] [ Google Scholar]
- Baker TP, Lenert JT, Parker J, Kemp B, Kushwaha A, Evans G. Lactating adenoma: a diagnosis of exclusion. Breast J 2001; 7(5):354-7. doi: 10.1046/j.1524-4741.2001.20075.x [Crossref] [ Google Scholar]
- Chico MJ, Causa Andrieu PI, Wernicke A, Pesce K. Breast lactating adenoma, an example of the utility of the radiological-pathological correlation. Clin Imaging 2021; 71:136-40. doi: 10.1016/j.clinimag.2020.11.009 [Crossref] [ Google Scholar]
- Hamadi H, Kiritta R, Ottoman O, Byabato S, Kihunrwa A, Rambau P. Spontaneous excessive bleeding from a breast lactating adenoma; a first reported case. EAS J Med Surg 2022; 4(2):36-40. doi: 10.36349/easjms.2022.v04i02.002 [Crossref] [ Google Scholar]
- Li D, Xiao X, Yang W, Shui R, Tu X, Lu H. Secretory breast carcinoma: a clinicopathological and immunophenotypic study of 15 cases with a review of the literature. Mod Pathol 2012; 25(4):567-75. doi: 10.1038/modpathol.2011.190 [Crossref] [ Google Scholar]
- Bussolati G, Tavassoli F, Nielsen B. Benign epithelial proliferations. In: Tavassoli FA, Devilee P, eds. Pathology and Genetics of Tumors of the Breast and Female Genital System. Lyon: IARC Press; 2000. p. 81-8.
- Jacob JD, Hodge C, Franko J, Pezzi CM, Goldman CD, Klimberg VS. Rare breast cancer: 246 invasive secretory carcinomas from the National Cancer Data Base. J Surg Oncol 2016; 113(7):721-5. doi: 10.1002/jso.24241 [Crossref] [ Google Scholar]