Arch Iran Med. 27(7):392-399.
doi: 10.34172/aim.28183
Systematic Review
Evaluation of Trans-Anal Endorectal Pull-Through Outcomes in Hirschsprung’s Disease in Different Age Groups: A Comprehensive Systematic Review
Farshid Ghasemi Meidansar Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, 1 
Mohammad Moradi Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing, 1 
Seyed Ali Nabipoorashrafi Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, 1
Seyyed Javad Nasiri Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, 2
Tahereh Chavoshi Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, 3
Mohammad Aldraji Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, 4
Fariba Jahangiri Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, 2, * 
Author information:
1Department of General Surgery, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
2Department of Pediatric Surgery, Ali-Asghar Children Hospital, Iran University of Medical Sciences, Tehran, Iran
3Department of Anesthesiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
4School of Medicine, Shahed University, Tehran, Iran
Abstract
Background:
The timing of trans-anal endorectal pull-through (TAEPT) for Hirschsprung’s disease (HD) is controversial. Early endorectal pull-through avoids the occurrence of preoperative enterocolitis. However, delayed pull-through (≥31 days) enables postnatal maturation of the anal canal and sphincter complex. The aim of this study was to identify the best age to perform trans-anal pull-through according to the literature.
Methods:
This is a comprehensive systematic review. All articles published from 2010 to 2022 were searched in the Web of Science, Ovid Medline, PubMed, CINAHIL, and Embase databases, using the keywords HD, delayed or early treatment, trans-anal pull-through surgery, age, sex or gender, complications and outcomes. Articles that met the inclusion criteria with good to fair quality according to the Newcastle-Ottawa quality assessment and low bias score in the Cochran collaboration tool were reviewed.
Results:
Sixteen studies were eligible to be reviewed. The overall results of this study showed that due to more common short-term complications at neonatal period and lower contrast enema diagnostic accuracy in determining the transition zone, it seems to be reasonable decision to postpone surgery until the child is several months old. There was also no difference in terms of complications and outcomes of trans-anal pull-through surgery between females and males.
Conclusion:
It is not recommended to delay surgery too much for ages over 1 year. Ages between 3 and 12 months can be a good time for interventional treatment for HD.
Keywords: Hirschsprung’s disease, Infant, Neonate, Systematic review, Trans-anal pull through
Copyright and License Information
© 2024 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Ghasemi Meidansar F, Moradi M, Nabipoorashrafi SA, Nasiri SJ, Chavoshi T, Aldraji M, et al. Evaluation of trans-anal endorectal pull-through outcomes in hirschsprung’s disease in different age groups: a comprehensive systematic review. Arch Iran Med. 2024;27(7):392-399. doi: 10.34172/aim.28183
Introduction
Hirschsprung’s disease (HD) is a pretty common surgical disease in children studied by pediatric surgeons and researchers. The prevalence of Hirschsprung is 1 per 5000 live births and the probability of transmission to the next generation is about 3%.1,2 This congenital disease is caused by a developmental disorder of the intestinal nervous system and is characterized by absence of ganglion cells in the submucosal layer (Meissner) and the myenteric network of the distal colon. The ganglion-free segment in the intestine lacks normal movement, so the proximal intestine dilates, leading to functional bowel obstruction, putting these patients at high risk for enterocolitis.3
Most cases of HD are now diagnosed in infancy. HD should be considered in neonates who do not have meconium excretion within 48 hours of birth or have vomiting and abdominal distention. Nonetheless, full-thickness biopsy of the rectal wall has been suggested as the most reliable test to endorse the diagnosis.4 Normal relaxation of the internal sphincter in response to rectal dilation is also impaired, which forms the basis of manometric diagnostic modality.5
Patients who have a confirmed diagnosis of HD undergo corrective surgery. In 1948, Swenson and Bill performed the first corrective surgery to remove the aganglionic segment of the colon followed by coloanal anastomosis.6,7 Traditionally, treatment involves a diverting colostomy at the time of diagnosis, and then, when the child grows older and weighs more than 10 kg, a definitive repair is considered. In 1998, De la Torre-Mondragón et al developed a single stage trans-anal pull-through for HD.8
In patients who respond to enema and rectal lavage, Botox can be used temporarily until the final pull-through operation is performed.9 In recent years, pull-through from the anal canal (TAEPT) has received much attention. In this method, laparotomy is not always required and the rectum is usually removed by maintaining the surrounding muscle cuff, leading to less damage to adjacent tissues and nerves.10,11
The advantages of TAEPT include easy technique, no need for colostomy, low bleeding rate and short hospital stay compared to other modalities. Nevertheless, there is no definite recommendation for the best age to perform the operation. Therefore, in this study, we investigated the best age group who are suitable to undergo corrective surgery with the fewest complications and best outcomes. Besides, the operation outcomes in males and females were compared.
Methods and Materials
This was a comprehensive systematic review. The present study included all studies regarding trans-anal pull-through in patients with HD. We included all studies evaluating this method in children with different age groups from 2010 to 2022. Non-English articles were excluded. Case reports, case series and expert opinions were also excluded. If necessary, the authors were contacted to provide more information. Moreover, we removed studies that did not report information regarding response to trans-anal pull-through surgery and its complications in Hirschsprung patients or did not have sufficient data. Five main databases were searched, including Web of Science, Ovid Medline, PubMed, CINAHIL, and Embase. The Google Scholar database was searched finally to ensure that the systematic search was complete. Abstracts published in conferences or dissertations on trans-anal pull-through in patients with Hirschsprung’s were also considered as much as possible. Studies that included patients with concurrent congenital abnormalities were excluded.
The main keywords included Hirschsprung’s, HD, congenital megacolon, trans-anal, pull-through, aganglionosis, aganglionic segment, aganglionic bowel, rectosigmoid colon, anorectal stenosis, enterocolitis, soiling, fecal continency, bowel continency, fecal soiling, pediatric, newborn, child, infant, infancy, neonate and Bowel obstruction.
Bias Assessment Tool
The bias of included studies was checked by two independent authors. In case of disagreement between the two authors, a consensus was reached through discussion and exchange of views or by requesting a third opinion. The Joanna Briggs Institute Critical Appraisal tool12 was used to assess the eligibility of studies. This tool consists of 10 questions in three main sections of design, conduct and analysis. Each question scores yes, no, unclear or not applicable. For instance, question No. 1 is “Was the sample representative of the target population?”12.
Data were extracted according to a standard protocol. The extracted information included study design, year of publication, authors’ name, sample size, surgical complications including enterocolitis, anastomotic stenosis, fecal continence and constipation, age and sex.
Results
Initially, 149 studies were found. After the primary review, duplicate studies (21 records) were excluded. After evaluating these 128 studies by their titles and abstracts, 61 articles were considered for full-text evaluation. Of these 61 studies, 45 studies were omitted for various reasons depicted in Figure 1, and 16 studies remained for the final analysis. The study flowchart is depicted in Figure 1. Our results were classified into two main categories including the results of trans-anal pull-through surgery based on age and gender.
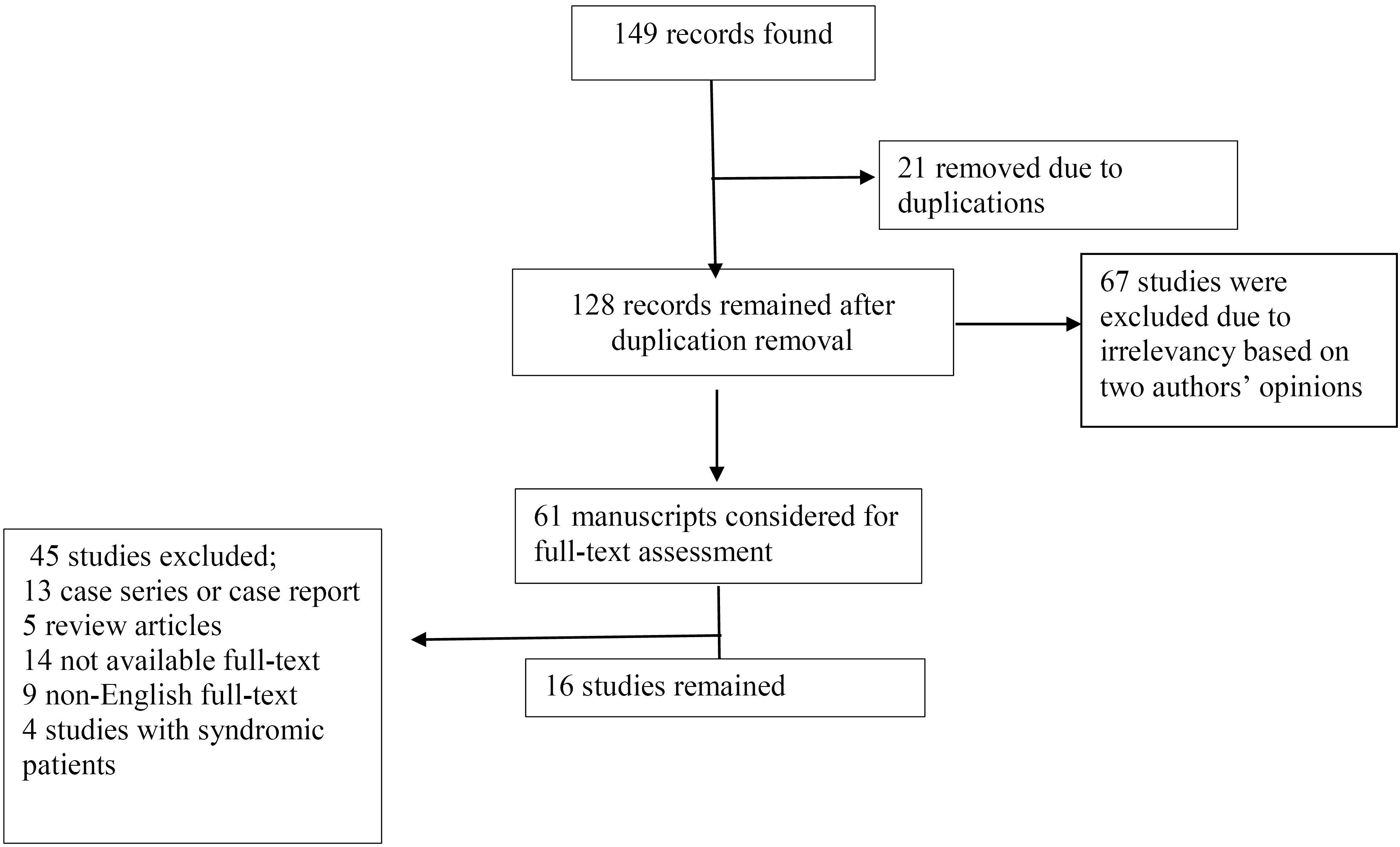
Figure 1.
The Study Flow Chart
.
The Study Flow Chart
Age
A large retrospective cohort study by Lu et al in both neonatal and non-neonatal groups (650 patients) found that a single-stage trans-anal pull-through in the non-neonatal period may be more appropriate than the neonatal period. There was a higher rate of perianal excoriation, anastomotic stenosis and leakage, postoperative enterocolitis, and postoperative incontinence in neonates compared to non-neonates.13 Furthermore, in another study by the same authors, Lu et al recommended home rectal irrigation, followed by a delayed and planned surgery, as intervention in the neonatal period lacks enough diagnostic accuracy and a higher rate of post-op enterocolitis.14 Moreover, in a retrospective cohort study in 2019, Zhu et al stated that children with Hirschsprung’s under 3 months of age had lower rates of accurate diagnostic results and poorer postoperative outcomes. They suggested that it may be more appropriate to wait until the child is above three months to perform surgery.15
A study by Freedman-Weiss et al on 282 patients found that for appropriately selected patients with HD, delaying pull-through until the second month of life is associated with lower total and postoperative stays without increased re-admissions or complications.16 However, in another study by Beltman et al, multivariate analysis showed that older age at surgery (median 105 days) increases the risk of developing postoperative complications (OR = 1.00, 95% CI = 1.00‒1.01, P = 0.041).17
On the other hand, Zakaria’s study compared two groups of patients regarding outcome and complications. The mean age in groups A and B was 14.02 ± 10.3 and 69.9 ± 32 months, respectively. They suggested that it may be better to perform surgery at a younger age and delaying surgery is associated with comorbidities. Most children at younger age showed no abnormal defecation problems, and had an excellent fecal continence score rate.18 Besides, in another study by the same author, fecal incontinence was less frequent when the operation was performed between 6 months to 2 years of age.19 Moreover, in Khalil’s study, higher age at surgery significantly affected physical function (β = -0.686, P = 0.001) and reduced school performance (β = -0.2279, P = 0.027). They indicated that age at surgery (patients’ age was 7-19 months) had a significant negative correlation with quality of life, as children who underwent surgery at a younger age had better quality of life.20
In another study by Kumar et al, the implications of the single-stage trans-anal endorectal pull-through (TAEPT) were assessed retrospectively. Perianal excoriation was reported in 60% of patients, more commonly in neonates. Besides, stool frequency was reported to be more than 5 to 7 per day in all neonates early after the procedure. Moreover, blood transfusion was higher in children above one year. They concluded that delaying surgery is not logical and neonates and infants might benefit the most compared to other age groups 21. Besides, Kastenberg et al compared delayed primary endorectal pull-through ( ≥ 31 days). The median age at operation was 98 days (IQR 61-188 days) for infants. They assess 82 patients, 49 neonates and 33 non-neonates. Fifteen neonates compared to five non-neonates developed fecal incontinence (P value = 0.13). Besides, enterocolitis and other complications were not different between the two groups.22
Furthermore, in a retrospective study by Dahal et al on 113 children, younger age at surgery (under 3 years) was associated with lower bowel frequency (less than 3 times a day) (P < 0.05). There was a significantly higher frequency of stool in patients aged more than 36 months and those with a resected colon more than 30 cm.23
On the other hand, a study by Miyano et al showed that age at surgery was not correlated with postoperative bowel function in HD.24 They reported that only operation duration was significantly higher in patients older than 4 years, and other outcomes did not differ significantly. Furthermore, in a study by Hoff et al, there was no risk factor for short-term complication using the Clavien-Dindo grading system, including age at surgery [median age 62 days] (OR = 2.97, 95% CI = 9.93‒0.92).25
Nonetheless, in a study by Byström et al, children who underwent TAEPT for Hirschsprung had significantly impaired bowel function scores compared with healthy controls in several aspects. There were no differences between age groups in this study [median age at TAEPT was 57 days (12‒3,355)], indicating impaired bowel function after TAEPT.26
On the other hand, in a multicenter study in Scandinavia to evaluate the predictors of functional outcomes, age at surgery did not have a significant effect on poor outcomes using a multivariate model.27 This study was a retrospective investigation mainly to identify long-term complications of children with HDs after operation. In addition, in a very recent study by Zhang et al, 229 neonates who underwent TAEPT were reviewed. They reported that operation in the neonatal period was quite safe with few complications (age of 6‒28 days).28 In 62 patients, there was no radiological transition zone (27.1%). Early post-op complications (wound infection, dehiscence, sepsis, etc) occurred in 26 patients (11.4%). Enterocolitis was noted in 16 (7%). The follow-up period ranged from 1.2 years to 14 years. Delayed complications (stricture, fistula, prolapse) were reported in 6 patients. Soiling persisted in 22 patients (20.8%).
Gender
Dahal et al reported the same incidence of postoperative complications in both males and females. There were no statistically significant differences in terms of stool frequency less than 3 times a day (male/female; 70%/100%, P = 0.09), soiling (male/female; 7%/12.5%, P = 0.4) and constipation (male/female; 3%/0%) 23. In another study, the prevalence of social problems after surgery was not affected by gender (P < 0.05).29 In addition, Dehghan et al compared the two methods of trans-abdominal or trans-anal pull-through in children with Hirschsprung, and reported no significant differences regarding complications in males and females.30 In another study, sphincter function was not related to the patient’s gender.31
In two different studies, there were no significant differences between males and females during the first 30 days after surgery in terms of Clavien-Dindo grading, anastomotic stenosis, postoperative enterocolitis, bleeding and wound infection, length of hospital stay after surgery, re-admission within 30 days after surgery, and the need for re-operation.25,26
In the study by Byström et al,26 the comparison of bowel function score between males and females with HD showed no significant difference. In another study 17, in univariate analysis, gender was not reported as a risk factor for postoperative complications (OR = 1.27, 95% CI = 0.38‒4.23, P = 0.698). In the study by Gunadi et al,32 no association between gender and voluntary bowel movement (VBM) was observed in Hirschsprung patients after TAEPT. A summary of main studies used in this systematic review and their outcomes is depicted in Table 1.
Table 1.
Summary of Studies Used in This Systematic Review
|
Author(s)
|
Publish Year
|
Subjects
|
Male
|
Female
|
Study Design
|
Quality
|
Outcome
|
| Lu et al13 |
2017 |
650 children in two groups of neonates and non-neonates |
497 |
153 |
Retrospective cohort |
Good |
TAEPT in the non-neonatal period may be more appropriate than in the neonatal period, especially regarding post-op complications. |
| Zhu et al15 |
2019 |
62 infants < 3 months and 136 infants aged 3-12 months |
158 |
40 |
Retrospective cohort |
Good |
Infants ≤ 3 months old with Hirschsprung's disease showed lower rates of accurate and conclusive diagnostic results and more postoperative complications. |
| Freedman-Weiss et al 16 |
2019 |
282 patients in two groups of < 31 days and 31-120 days old |
231 |
51 |
Retrospective cohort |
Good |
Delaying pull-through until the second month of life is associated with lower total and postoperative stays without increased readmissions or complications. |
| Beltman et al17 |
2021 |
106 patients underwent TAEPT (Median age at time of surgery:105 days) |
80 |
26 |
Retrospective |
Good |
Older age at time of surgery was a risk factor for postoperative complications. |
| Zakaria 18 |
2012 |
40 patients in two age groups (6-42 months and 3.5-13 years old) |
28 |
12 |
Comparative retrospective cohort |
Good |
Group A had fewer defecation problems, and had excellent fecal continence scores. |
| Zakaria et al 19 |
2012 |
50 patients in two age groups |
27 |
23 |
Retrospective cohort |
Good |
The earlier the surgery of HD, the lower the incidence of fecal incontinence
(6 months - 2 years had better results than > 2 years). |
| Khalil et al 20 |
2015 |
70 patients |
37 |
16 |
Retrospective |
Fair |
Surgery at a younger age (patient age 7-19 months) is associated with better quality of life. |
| Kumar et al 21 |
2019 |
30 patients including 10 neonates, 13 infants and 7 children |
26 |
4 |
Retrospective/Prospective cohort |
Good |
Excoriation was higher in neonates, but overall outcomes were better in neonates. |
| Kastenberg et al 22 |
2021 |
82 patients |
68 |
14 |
Retrospective |
Good |
Fecal incontinence more frequent in neonates (P value = 0.13), but overall equivalent outcomes. |
| Dahal et al 23 |
2011 |
131 children with HD aged 7 days to 14 years |
112 |
19 |
Retrospective |
Good |
Younger age at surgery (under 3 years) was associated with lower bowel frequency (less than 3 times a day). |
| Miyano et al 24 |
2017 |
106 patients underwent laparoscopic pull-through in 4 age groups ( < 3 months, 3-11 months, 1-3 years and > 3 years) |
68 |
38 |
Prospective cohort |
Good |
Age at surgery was not correlated with postoperative bowel function in Hirschsprung's disease. |
| Hoff et al 25 |
2019 |
69 patients |
51 |
18 |
Cohort |
Fair |
There was no risk factor for short-term Clavien-Dindo complication, including age at surgery [median age 62 days]. |
| Byström et al26 |
2020 |
30 Hirschsprung patients treated with TAEPT and 30 healthy controls matched for age and gender |
22 |
8 |
Cross-sectional case–control study |
Good |
Post-operative BFS (bowel function score) did not show a significant difference in Hirschsprung patients between age groups.[median age at TAEPT was 57 days (12-3,355)] |
| Bjørnland et al 27 |
2017 |
200 patients |
169 |
31 |
Retrospective |
Fair |
Age at the time of operation (median age 3 months) does not affect the frequency of poor outcomes (stoma, appendicostomy, daily fecal accidents or use of regular enemas). |
| Yanan Zhang et al28 |
2022 |
229 neonates |
187 |
42 |
Cross-sectional |
Good |
Operation in the neonatal period was quite safe with few complications (age 6-28 days). |
| Stensrud et al 31 |
2015 |
52 patients |
42 |
10 |
Prospective cohort |
Good |
Internal anal sphincter defects occurred more often in younger children(median age 1.8 month) |
Discussion
The consequences of TAEPT surgery for HD are not always as favorable as the surgeon imagines. Incomplete continence, constipation and postoperative enterocolitis should not be ignored.33-36 Previous studies have attempted to investigate the relationship between preoperative characteristics and surgical outcomes in patients with Hirschsprung’s such as age, gender, length of aganglionosis, age at surgery, preoperative enterocolitis, comorbidities, and genetic background.37-40 Nevertheless, there is still controversy regarding the best age of operation. Therefore, here, we mainly tried to categorize studies that prefer the neonatal period as the best age against those who believe to postpone it. We mostly focused on 16 studies according to our inclusion and exclusion criteria. In summary, 11 investigations were in favor of postponing the operation to an age above one month, 2 studies found no difference and 3 reported better outcomes in the neonatal period.
One important issue here is the accuracy of diagnostic modalities for detecting patients. A recent study by Chen et al showed 88.5% correlation between radiological and pathological TZ in rectosigmoid Hirschsprung. This was dependent on the patient’s age. They showed 69% correlation for children under 3 months versus 85.3% (high severity) for older ones.41 Overall, the different studies mentioned above indicated that longer periods of disease may lead to better development of radiological TZ. Therefore, determining the transition zone with a high accuracy is necessary. Most children with HD present during the neonatal period with delayed passage of meconium beyond the first 24 hours, abdominal distention, bilious vomiting and feeding intolerance and are diagnosed by a rectal biopsy in the first month of life. However, a definite diagnosis before surgery is mandatory.
The extent of colon caliber changes depends on the duration of distal bowel obstruction, which is limited in newborns. Therefore, contrast enema is not appropriate in newborns. This is the reason why some surgeons prefer to wait for 1‒2 months. A colon enema performed before the age of 30 days had a sevenfold higher probability of false-negative results.42
The main risk of postponing surgery is the possibility of enterocolitis during the waiting period. This risk can be lowered by ensuring rectal pressure relief with adequate irrigation (usually 10‒20 mL/kg, several times daily), administration of prophylactic metronidazole or probiotics. Due to the risk of enterocolitis, many pediatric surgeons believe that once a diagnosis is made, even in small infants, a laparoscopic or trans-anal operation can be performed successfully and safely.43 A survey by the European Society of Pediatric Surgeons found that 33% of pediatric surgeons prefer to perform endorectal pull-through surgery at diagnosis and 67% prefer a delayed approach (4 months or > 5 kg).44
In addition, anorectal manometry is an effective and safe method that complements the diagnosis of HD in newborns. Anorectal sphincter pressure progressively matures with incremental increase during the first months of life 45-47.
Kaiser Decker et al reported that a rectal suction biopsy (RSB) had 81% sensitivity and 97% specificity. Therefore, repeated sampling may be necessary. They found that RSB can also be reliable and safely performed in preterm infants.48 However, repeated biopsies in neonates may lead to intestinal perforation. In the study by Putnam et al in clinically suspicious neonates for HD, contrast enema studies showed inconclusive results in 32% of cases.49
Kumar et al concluded that delaying surgery is not logical, and neonates and infants might benefit the most compared to other age groups. As their study design was retrospective with a small number of patients in each sub-group, they could not provide a detailed comparison between neonates and those between 1-12 months. However, the risk of reported complications was higher in neonates.21
Besides, Kastenberg et al compared delayed primary endorectal pull-through ( ≥ 31 days). The median age at operation was 98 days (IQR 61 - 188 days) for patients. They assess 82 patients, 49 neonates and 33 non-neonates. Fifteen neonates compared to five non-neonates developed fecal incontinence (P value = 0.13). Besides, enterocolitis and other complications were not different between the two groups. As fecal incontinence was more frequent in neonates, but without a statistically significant difference, the authors tended to conclude that operation in neonates is as safe as those above one month, which is not logical in our opinion. This study had a good methodological design but with a small sample size. Therefore, we might not rely completely on this analysis to advocate surgery in neonates.22
In another investigation, Karlsen et al compared the outcomes of laparoscopic and trans-anal pull-through and reported poorer outcomes in the neonatal period. This study did not fulfill our inclusion criteria but higher complications were reported in neonates.50
In another study by Ivana et al,32 no association was found between age at surgery and functional outcomes in Hirschsprung patients. We believe this study lacks a large sample size. Also, they categorized their patients into two groups of those under 4 years and those above, which ignores any classification regarding neonates; therefore, the results might not be very helpful in our data interpretation. Nevertheless, soiling was more frequently reported in patients older than 4 years which is consistent with some other reports. Accordingly, delaying surgery above 2‒4 years is completely erroneous.
On the other hand, the study by Miyano et al showed that age at surgery was not correlated with postoperative bowel function in HD. However, their emphasis was to validate and highlight their modified laparoscopic technique for HD and their main goal was not comparison between neonates and non-neonates regarding outcomes.24
In addition, Hoff et al reported no risk factor for short-term complication using the Clavien-Dindo grading system, including age at surgery.25 They only evaluated outcomes during the first months after surgery as early post-operation complications, and long-term outcomes were notassessed.
On the other hand, in a multicenter study in Scandinavia to evaluate the predictors of functional outcomes, age at surgery did not have a significant effect on poor outcomes using a multivariate model.27 This study was a retrospective investigation mainly to identify long-term complications of children with HDs after operation. Age classification in this study was only given in a table categorized as 0.4 to 1, 1‒2.9, 3‒7.5 and 7.9‒133 months. No detailed information regarding the number of patients in each quartile was found. Their main goal was to assess long-term bowel function and they did not compare complications across different age groups. Therefore, we cannot conclude that the results contradict our recommendation.
The most reliable study against postponing the operation to above one month was the one conducted by Zhang et al on 229 neonates.28 They reported that operation in the neonatal period was quite safe with few complications (age 6‒28 days). This is almost the only well-organized study to defend operation in the neonatal period with a quite large sample size. We admire the authors for the settings they prepared in their study. Nevertheless, we believe that these outcomes are due to the high expertise of the staff in this center as they could recruit 229 patients in 13 years. As most studies reported smaller numbers of patients, we believe delaying the operation to above one month is logical in smaller centers. However, in very few tertiary centers with highly organized settings and experienced pediatric surgeons, we might recommend surgery in neonates.
In terms of postoperative complications, infants who undergo trans-anal pull-through surgery are exposed to undesirable short-term consequences. Huang et al 51 reported that neonates have a longer recovery period after surgery compared to non-neonates. Furthermore, active immunodeficiency and inactive immunity of maternal antibodies result in low resistance to infection in neonates. In a retrospective study evaluating the results of a single-stage trans-anal pull-through in 650 children, the authors concluded that the operation might be more appropriate in the non-neonatal period compared to the neonatal period. Because there was a higher rate of perianal excoriation, anastomotic stenosis and leakage, postoperative enterocolitis and incomplete postoperative continence in neonates than non-neonates.13 In addition, Stensrud et al31 compared two groups of patients who underwent trans-anal and trans-abdominal surgery regarding anal sphincter damage using ultrasonography. They showed that children who underwent trans-anal pull-through had higher rates of injury. The median age of patients in trans-anal and trans-abdominal surgery was 1.8 (0.4‒133) and 13 (1.2‒100) months. We might conclude that operation at lower ages and using the trans-anal approach would increase the likelihood of sphincter injury.
A meta-analysis by Westfal et al 52 published in 2021 included four studies in addition to their own center’s data to assess the best time to perform the operation for children with HD. They included the findings of Miyano et al,24 Zhu et al,15 Lu et al14 and Chung et al53 in their pooled analysis. We discussed the first three above; however, the study by Chung et al53 could not be entered because they did not include a clear age classification to separate between neonates and non-neonates. However, as they provided their data sheet, Westfal et al could include it. Overall, Westfal et al claimed that children below 2.5 months of age at surgery would have poorer outcomes, which is somehow in line with the results of our systematic review.
This study had some limitations. As all systematic reviews, we had to rely on information from other studies. A recall bias is inevitable when compiling information from other investigations. Some studies did not include necessary information needed for our review. Therefore, some high-quality studies might have been excluded due to a high bias score according to the checklist. Besides, some studies included patients with concurrent syndromes which were not assessed in our review. We do not know whether Down syndrome might affect the decision for age selection. We suggest large multicentric studies to collect data on different ethnicities.
Conclusion
Despite the recommendation of most studies to treat HD as early as possible, due to more common short-term complications and lower contrast enema diagnostic accuracy in neonates, it seems a reasonable decision to postpone surgery until the child is several months old. However, it is not recommended to delay surgery over 1 year. The overall view on most reviewed articles indicates that age between 3 and 12 months can be a good time for interventional treatment for HD. However, we cannot complain performing surgery in high-volume advanced tertiary centers in the neonatal period.
Competing Interests
We declare no conflict of interest.
Data Availability Statement
Data could be provided in case of need.
Ethical Approval
Not applicable.
Funding
This was part of a thesis to obtain a specialist’s degree in general surgery by one of the authors and supported by Iran University of Medical Sciences (IUMS).
References
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet 2008; 45(1):1-14. doi: 10.1136/jmg.2007.053959 [Crossref] [ Google Scholar]
- Anderson JE, Vanover MA, Saadai P, Stark RA, Stephenson JT, Hirose S. Epidemiology of Hirschsprung disease in California from 1995 to 2013. Pediatr Surg Int 2018; 34(12):1299-303. doi: 10.1007/s00383-018-4363-9 [Crossref] [ Google Scholar]
- Amiel J, Lyonnet S. Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet 2001; 38(11):729-39. doi: 10.1136/jmg.38.11.729 [Crossref] [ Google Scholar]
- Ambartsumyan L, Smith C, Kapur RP. Diagnosis of Hirschsprung disease. Pediatr Dev Pathol 2020; 23(1):8-22. doi: 10.1177/1093526619892351 [Crossref] [ Google Scholar]
- de Lorijn F, Kremer LC, Reitsma JB, Benninga MA. Diagnostic tests in Hirschsprung disease: a systematic review. J Pediatr Gastroenterol Nutr 2006; 42(5):496-505. doi: 10.1097/01.mpg.0000214164.90939.92 [Crossref] [ Google Scholar]
- Haricharan RN, Georgeson KE. Hirschsprung disease. Semin Pediatr Surg 2008; 17(4):266-75. doi: 10.1053/j.sempedsurg.2008.07.005 [Crossref] [ Google Scholar]
- Levitt MA, Hamrick MC, Eradi B, Bischoff A, Hall J, Peña A. Transanal, full-thickness, Swenson-like approach for Hirschsprung disease. J Pediatr Surg 2013; 48(11):2289-95. doi: 10.1016/j.jpedsurg.2013.03.002 [Crossref] [ Google Scholar]
- De la Torre-Mondragón L, Ortega-Salgado JA. Transanal endorectal pull-through for Hirschsprung’s disease. J Pediatr Surg 1998; 33(8):1283-6. doi: 10.1016/s0022-3468(98)90169-5 [Crossref] [ Google Scholar]
- Hosseini SM, Foroutan HR, Zeraatian S, Sabet B. Botulinium toxin, as bridge to transanal pull-through in neonate with Hirschsprung’s disease. J Indian Assoc Pediatr Surg 2008; 13(2):69-71. doi: 10.4103/0971-9261.43025 [Crossref] [ Google Scholar]
- Wang JX, Dahal GR. Hirschsprung’s disease management: from multi staged operation to single-staged transanal pull-through. Nepal Med Coll J 2009; 11(2):138-42. [ Google Scholar]
- Ishikawa N, Kubota A, Kawahara H, Hasegawa T, Okuyama H, Uehara S. Transanal mucosectomy for endorectal pull-through in Hirschsprung’s disease: comparison of abdominal, extraanal and transanal approaches. Pediatr Surg Int 2008; 24(10):1127-9. doi: 10.1007/s00383-008-2231-8 [Crossref] [ Google Scholar]
- Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 2014; 3(3):123-8. doi: 10.15171/ijhpm.2014.71 [Crossref] [ Google Scholar]
- Lu C, Hou G, Liu C, Geng Q, Xu X, Zhang J. Single-stage transanal endorectal pull-through procedure for correction of Hirschsprung disease in neonates and nonneonates: a multicenter study. J Pediatr Surg 2017; 52(7):1102-7. doi: 10.1016/j.jpedsurg.2017.01.061 [Crossref] [ Google Scholar]
- Lu C, Xie H, Li H, Geng Q, Chen H, Mo X. Feasibility and efficacy of home rectal irrigation in neonates and early infancy with Hirschsprung disease. Pediatr Surg Int 2019; 35(11):1245-53. doi: 10.1007/s00383-019-04552-8 [Crossref] [ Google Scholar]
- Zhu T, Sun X, Wei M, Yi B, Zhao X, Wang W. Optimal time for single-stage pull-through colectomy in infants with short-segment Hirschsprung disease. Int J Colorectal Dis 2019; 34(2):255-9. doi: 10.1007/s00384-018-3179-3 [Crossref] [ Google Scholar]
- Freedman-Weiss MR, Chiu AS, Caty MG, Solomon DG. Delay in operation for Hirschsprung disease is associated with decreased length of stay: a 5-year NSQIP-Peds analysis. J Perinatol 2019; 39(8):1105-10. doi: 10.1038/s41372-019-0405-y [Crossref] [ Google Scholar]
- Beltman L, Roorda D, Backes M, Oosterlaan J, van Heurn LW, Derikx JP. Risk factors for short-term complications graded by Clavien-Dindo after transanal endorectal pull-through in patients with Hirschsprung disease. J Pediatr Surg 2022; 57(8):1460-6. doi: 10.1016/j.jpedsurg.2021.07.024 [Crossref] [ Google Scholar]
- Zakaria OM. Bowel function and fecal continence after Soave’s transanal endorectal pull-through for Hirschsprung’s disease: a local experience. Updates Surg 2012; 64(2):113-8. doi: 10.1007/s13304-012-0140-9 [Crossref] [ Google Scholar]
- Zakaria OM, El Labban GM, Shams ME. Fecal incontinence after single-stage Soave’s pull-through: abdominal versus transanal endorectal pull-through. Ann Pediatr Surg 2012; 8(1):5-8. doi: 10.1097/01.xps.0000407759.30719.57 [Crossref] [ Google Scholar]
- Khalil M. Long-term health-related quality of life for patients with Hirschsprung’s disease at 5 years after transanal endorectal pull-through operation. Qual Life Res 2015; 24(11):2733-8. doi: 10.1007/s11136-015-1012-9 [Crossref] [ Google Scholar]
- Senthil Kumar L, Jayaprakash S, Rajamani G. Single-stage transanal endorectal pull-through (TEPT) for Hirschsprung’s disease. IOSR J Dent Med Sci 2012; 18(7):1-12. doi: 10.9790/0853-1807040112 [Crossref] [ Google Scholar]
- Kastenberg ZJ, Taylor MA, Durham MM, Calkins CM, Rentea RM, Wood RJ. Perioperative and long-term functional outcomes of neonatal versus delayed primary endorectal pull-through for children with Hirschsprung disease: a pediatric colorectal and pelvic learning consortium study. J Pediatr Surg 2021; 56(8):1465-9. doi: 10.1016/j.jpedsurg.2021.04.024 [Crossref] [ Google Scholar]
- Dahal GR, Wang JX, Guo LH. Long-term outcome of children after single-stage transanal endorectal pull-through for Hirschsprung’s disease. World J Pediatr 2011; 7(1):65-9. doi: 10.1007/s12519-011-0247-y [Crossref] [ Google Scholar]
- Miyano G, Takeda M, Koga H, Okawada M, Nakazawa-Tanaka N, Ishii J. Hirschsprung’s disease in the laparoscopic transanal pull-through era: implications of age at surgery and technical aspects. Pediatr Surg Int 2018; 34(2):183-8. doi: 10.1007/s00383-017-4187-z [Crossref] [ Google Scholar]
- Hoff N, Wester T, Granström AL. Classification of short-term complications after transanal endorectal pull-through for Hirschsprung’s disease using the Clavien-Dindo-grading system. Pediatr Surg Int 2019; 35(11):1239-43. doi: 10.1007/s00383-019-04546-6 [Crossref] [ Google Scholar]
- Byström C, Östlund S, Hoff N, Wester T, Granström AL. Evaluation of bowel function, urinary tract function, and quality of life after transanal endorectal pull-through surgery for Hirschsprung’s disease. Eur J Pediatr Surg 2021; 31(1):40-8. doi: 10.1055/s-0040-1715612 [Crossref] [ Google Scholar]
- Bjørnland K, Pakarinen MP, Stenstrøm P, Stensrud KJ, Neuvonen M, Granström AL. A Nordic multicenter survey of long-term bowel function after transanal endorectal pull-through in 200 patients with rectosigmoid Hirschsprung disease. J Pediatr Surg 2017; 52(9):1458-64. doi: 10.1016/j.jpedsurg.2017.01.001 [Crossref] [ Google Scholar]
- Zhang Y, Liu Z, Li S, Yang S, Zhao J, Yang T. One-stage transanal endorectal pull-through for Hirschsprung disease: experience with 229 neonates. Pediatr Surg Int 2022; 38(11):1533-40. doi: 10.1007/s00383-022-05198-9 [Crossref] [ Google Scholar]
- Neuvonen MI, Kyrklund K, Rintala RJ, Pakarinen MP. Bowel function and quality of life after transanal endorectal pull-through for Hirschsprung disease: controlled outcomes up to adulthood. Ann Surg 2017; 265(3):622-9. doi: 10.1097/sla.0000000000001695 [Crossref] [ Google Scholar]
- Dehghan A, Hosseini SM, Rahimi A, Zare S, Khazdooz M, Khoshnavaz R, et al. Transanal endo rectal pull-through versus trans abdominal pull-through in management of Hirschsprung’s disease. Hormozgan Med J 2013;17(1):1-7. [Persian].
- Stensrud KJ, Emblem R, Bjørnland K. Anal endosonography and bowel function in patients undergoing different types of endorectal pull-through procedures for Hirschsprung disease. J Pediatr Surg 2015; 50(8):1341-6. doi: 10.1016/j.jpedsurg.2014.12.024 [Crossref] [ Google Scholar]
- Gunadi Gunadi, Ivana G, Mursalin DA, Pitaka RT, Zain MW, Puspitarani DA. Functional outcomes of patients with short-segment Hirschsprung disease after transanal endorectal pull-through. BMC Gastroenterol 2021; 21(1):85. doi: 10.1186/s12876-021-01668-x [Crossref] [ Google Scholar]
- More K, Rao S, McMichael J, Minutillo C. Growth and developmental outcomes of infants with Hirschsprung disease presenting in the neonatal period: a retrospective study. J Pediatr 2014;165(1):73-7.e2. 10.1016/j.jpeds.2014.02.062.
- Aworanti OM, McDowell DT, Martin IM, Hung J, Quinn F. Comparative review of functional outcomes post-surgery for Hirschsprung’s disease utilizing the paediatric incontinence and constipation scoring system. Pediatr Surg Int 2012; 28(11):1071-8. doi: 10.1007/s00383-012-3170-y [Crossref] [ Google Scholar]
- Stensrud KJ, Emblem R, Bjørnland K. Functional outcome after operation for Hirschsprung disease--transanal vs transabdominal approach. J Pediatr Surg 2010; 45(8):1640-4. doi: 10.1016/j.jpedsurg.2010.02.065 [Crossref] [ Google Scholar]
- Gunnarsdóttir A, Sandblom G, Arnbjörnsson E, Larsson LT. Quality of life in adults operated on for Hirschsprung disease in childhood. J Pediatr Gastroenterol Nutr 2010; 51(2):160-6. doi: 10.1097/MPG.0b013e3181cac1b6 [Crossref] [ Google Scholar]
- Pini Prato A, Gentilino V, Giunta C, Avanzini S, Mattioli G, Parodi S. Hirschsprung disease: do risk factors of poor surgical outcome exist?. J Pediatr Surg 2008; 43(4):612-9. doi: 10.1016/j.jpedsurg.2007.10.007 [Crossref] [ Google Scholar]
- Catto-Smith AG, Trajanovska M, Taylor RG. Long-term continence after surgery for Hirschsprung’s disease. J Gastroenterol Hepatol 2007; 22(12):2273-82. doi: 10.1111/j.1440-1746.2006.04750.x [Crossref] [ Google Scholar]
- Catto-Smith AG, Trajanovska M, Taylor RG. Long-term continence in patients with Hirschsprung’s disease and Down syndrome. J Gastroenterol Hepatol 2006; 21(4):748-53. doi: 10.1111/j.1440-1746.2005.03996.x [Crossref] [ Google Scholar]
- Engum SA, Grosfeld JL. Long-term results of treatment of Hirschsprung’s disease. Semin Pediatr Surg 2004; 13(4):273-85. doi: 10.1053/j.sempedsurg.2004.10.015 [Crossref] [ Google Scholar]
- Chen X, Xiaojuan W, Zhang H, Jiao C, Yu K, Zhu T. Diagnostic value of the preoperatively detected radiological transition zone in Hirschsprung’s disease. Pediatr Surg Int 2017; 33(5):581-6. doi: 10.1007/s00383-017-4064-9 [Crossref] [ Google Scholar]
- Frongia G, Günther P, Schenk JP, Strube K, Kessler M, Mehrabi A. Contrast enema for Hirschsprung disease investigation: diagnostic accuracy and validity for subsequent diagnostic and surgical planning. Eur J Pediatr Surg 2016; 26(2):207-14. doi: 10.1055/s-0035-1546755 [Crossref] [ Google Scholar]
- Gosain A, Frykman PK, Cowles RA, Horton J, Levitt M, Rothstein DH. Guidelines for the diagnosis and management of Hirschsprung-associated enterocolitis. Pediatr Surg Int 2017; 33(5):517-21. doi: 10.1007/s00383-017-4065-8 [Crossref] [ Google Scholar]
- Zani A, Eaton S, Morini F, Puri P, Rintala R, Heurn EV. European Paediatric Surgeons’ Association survey on the management of Hirschsprung disease. Eur J Pediatr Surg 2017; 27(1):96-101. doi: 10.1055/s-0036-1593991 [Crossref] [ Google Scholar]
- Huang Y, Zheng S, Xiao X. Preliminary evaluation of anorectal manometry in diagnosing Hirschsprung’s disease in neonates. Pediatr Surg Int 2009; 25(1):41-5. doi: 10.1007/s00383-008-2293-7 [Crossref] [ Google Scholar]
- Tang YF, Chen JG, An HJ, Jin P, Yang L, Dai ZF. High-resolution anorectal manometry in newborns: normative values and diagnostic utility in Hirschsprung disease. Neurogastroenterol Motil 2014; 26(11):1565-72. doi: 10.1111/nmo.12423 [Crossref] [ Google Scholar]
- Enríquez Zarabozo E, Núñez Núñez R, Ayuso Velasco R, Vargas Muñoz I, Fernández de Mera JJ, Blesa Sánchez E. [Anorectal manometry in the neonatal diagnosis of Hirschsprung’s disease]. Cir Pediatr 2010;23(1):40-5. [Spanish].
- Keyzer-Dekker CM, Sloots CE, Schokker-van Linschoten IK, Biermann K, Meeussen C, Doukas M. Effectiveness of rectal suction biopsy in diagnosing Hirschsprung disease. Eur J Pediatr Surg 2016; 26(1):100-5. doi: 10.1055/s-0035-1566099 [Crossref] [ Google Scholar]
- Putnam LR, John SD, Greenfield SA, Kellagher CM, Austin MT, Lally KP. The utility of the contrast enema in neonates with suspected Hirschsprung disease. J Pediatr Surg 2015; 50(6):963-6. doi: 10.1016/j.jpedsurg.2015.03.019 [Crossref] [ Google Scholar]
- Karlsen RA, Hoel AT, Fosby MV, Ertresvåg K, Austrheim AI, Stensrud KJ. Comparison of clinical outcomes after total transanal and laparoscopic assisted endorectal pull-through in patients with rectosigmoid Hirschsprung disease. J Pediatr Surg 2022; 57(9):69-74. doi: 10.1016/j.jpedsurg.2022.01.011 [Crossref] [ Google Scholar]
- Huang B, Li WM, Feng ZY, Huang LY. [Outcomes and defecation after one-stage transanal endorectal pull-through procedure for Hirschsprung disease]. Zhonghua Wei Chang Wai Ke Za Zhi 2012; 15(7):715-8. [ Google Scholar]
- Westfal ML, Okiemy O, Chung PH, Feng J, Lu C, Miyano G. Optimal timing for Soave primary pull-through in short-segment Hirschsprung disease: a meta-analysis. J Pediatr Surg 2022; 57(4):719-25. doi: 10.1016/j.jpedsurg.2021.07.007 [Crossref] [ Google Scholar]
- Chung PH, Wong KK, Tam PK, Leung MW, Chao NS, Liu KK. Are all patients with short segment Hirschsprung’s disease equal? A retrospective multicenter study. Pediatr Surg Int 2018; 34(1):47-53. doi: 10.1007/s00383-017-4202-4 [Crossref] [ Google Scholar]