Arch Iran Med. 27(4):206-215.
doi: 10.34172/aim.2024.30
Original Article
Neoadjuvant Chemotherapy in Patients with HER2-Negative Breast Cancer: A Report from Clinical Breast Cancer Registry of Iran
Kamran Roudini Conceptualization, Supervision, Writing – review & editing, 1 
Mehrzad Mirzania Conceptualization, Supervision, 1
Tahereh Yavari Writing – original draft, Writing – review & editing, 2
Monireh Sadat Seyyedsalehi Methodology, 3, 4
Azin Nahvijou Methodology, 3
Jayran Zebardast Formal analysis, Methodology, 5, 6
Mina Saadat Writing – original draft, 7
Ahmad Khajeh-Mehrizi Conceptualization, Formal analysis, Writing – review & editing, 1, * 
Author information:
1Department of Hematology and Medical Oncology, Cancer Institute, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
2Department of Internal Medicine, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
3Cancer Research Center, Cancer Institute, Tehran University of Medical Sciences, Tehran, Iran
4Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy
5Department of Cognitive Linguistics, Institute for Cognitive Science Studies (ICSS), Tehran, Iran
6Advanced Diagnostic and Interventional Radiology Research Center (ADIR), Tehran University of Medical Science, Tehran, Iran
7Student Research Committee, School of Nursing and Midwifery, Shahroud University of Medical Science, Shahroud, Iran
Abstract
Background:
Neoadjuvant chemotherapy (NCT) has become an increasingly popular approach in management of breast cancer (BC). This study was conducted to evaluate the pathologic response and 36-month recurrence and survival rates of patients with human epidermal growth factor receptor 2 (HER2)-negative BC treated with different NCT regimens.
Methods:
A total of 163 female patients with HER2-negative BC who received NCT during 2017-2020 were identified from the Clinical Breast Cancer Registry of Iran and entered the study. The prescribed NCT regimens included 4 cycles of doxorubicin plus cyclophosphamide, 4 cycles of doxorubicin plus cyclophosphamide followed by 4 cycles of paclitaxel, 4 cycles of doxorubicin plus cyclophosphamide followed by 4 cycles of docetaxel or 6 cycles of doxorubicin plus cyclophosphamide plus docetaxel (TAC).
Results:
Thirty-two patients (19.6%) experienced pathologic complete response (pCR). TAC regimen, triple negative-BC and ki67>10% were significantly associated with increased pCR. The recurrence, overall survival (OS) and disease-free survival (DFS) rate at 36 months for all patients were 16.6%, 84.7% and 79.8%, respectively. Type of neoadjuvant regimen as well as age, hormone receptor status, Ki67, grade, clinical stage, type of surgery and pathologic response to chemotherapy did not significantly influence the survival and recurrence; however, TAC results in improved recurrence, OS and DFS rates.
Conclusion:
This study provides further evidence that NCT is a viable treatment option for patients with HER2-negative BC. The TAC regimen resulted in a significantly higher pCR rate compared to other regimens, but did not result in a significant improvement in recurrence, OS and DFS and rates.
Keywords: Breast cancer, HER2-negative, Pathologic complete response, Recurrence, Survival
Copyright and License Information
© 2024 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Roudini K, Mirzania M, Yavari T, Seyyedsalehi MS, Nahvijou A, Zebardast J, et al. Neoadjuvant chemotherapy in patients with HER2-negative breast cancer: a report from clinical breast cancer registry of Iran. Arch Iran Med. 2024;27(4):206-215. doi: 10.34172/aim.2024.30
Introduction
Breast cancer (BC) is the most common malignancy in women.1 Globally, BC accounts for 25% of all types of cancers including 1.7 million new cases per year.2 It is a complex and heterogeneous disease, and its treatment often involves a combination of surgery, radiation therapy, and systemic therapy.3,4 The treatment of BC can have a significant impact on the patient’s quality of life.5 Recent efforts to provide new treatments for BC have focused on targeted therapies, immunotherapy, and combination therapies, as well as the development of novel drug delivery systems.6-8
Neoadjuvant chemotherapy (NCT), commonly prescribed before a surgery, has become an increasingly popular approach for treating BC.9,10 It allows for the evaluation of tumor response to chemotherapy and may improve the chances of breast-conserving surgery.11-14 Also, NCT affects the tumor microenvironment and immune response reflecting the potential for the development of novel treatment strategies.15 It has been reported that NCT provides the opportunity for a more individualized approach to treatment and can lead to improved outcomes, particularly in patients with locally advanced or inflammatory BC.16,17
Various medications are prescribed for NCT of BC, including anthracyclines, taxanes, and platinum-based agents.18 Platinum-based drugs, such as cisplatin and carboplatin, inhibit DNA synthesis and ultimately lead to apoptosis.19 Anthracyclines, such as doxorubicin and epirubicin, are potent cytotoxic agents that interfere with DNA replication and induce cell death.20 Taxanes, such as paclitaxel and docetaxel, exert their anti-cancer effects by stabilizing microtubules and disrupting the normal cell division process.21 To optimize treatment outcomes, these agents are often used together or alongside targeted therapies, like human epidermal growth factor receptor 2 (HER2) inhibitors or cyclin-dependent kinase 4 and 6 (CDK 4/6) Inhibitors.22 Tumor size, Ki67 percent, HER2, hormone (estrogen and progesterone) receptor, and overall health status are considered in the selection of specific chemotherapeutic agents and dosing regimens.23 Ki67 percent is a measure of cancer cell proliferation and can predict how cancer cells will respond to certain treatments.3 HER2 inhibitors should be used in conjunction with chemotherapy for BC with HER2 amplification, while BC without HER2 amplification (HER2 negative) does not require these agents.4 HER2 negative BC can be classified by the presence of hormone receptors, and those without hormone receptors are called triple-negative breast cancers (TNBCs) which tend to be more aggressive and have fewer targeted treatment options compared to other BC subtypes.23
Meanwhile, the use of NCT for BC treatment has been a subject of debate, with conflicting reports regarding the effects on patient outcomes and survival.24,25 Some studies have demonstrated that NCT can result in significant tumor shrinkage, allowing for less invasive surgical interventions and improved patient outcomes.26,27 However, other studies have suggested that the use of NCT does not provide significant survival benefits over traditional adjuvant chemotherapy (ACT).24,28 Moreover, concerns have been raised about the potential for NCT to increase the risk of disease recurrence, particularly in patients with TNBC.24,29,30 Studies have shown that various NCT regimens have different effects on the response rate of patients with BC.25,31,32
Despite these controversies, NCT remains an important treatment option for BC, and ongoing research is focused on identifying patient subgroups who may benefit the most from this approach.24 This study was conducted to evaluate the three-year survival of patients with HER2 negative BC who underwent NCT. We also compared the pathologic response rate, recurrence and mortality among patients treated with different NCT regimens.
Materials and Methods
All patients newly diagnosed with BC during 2017–2020 and treated with NCT were identified from the Clinical Breast Cancer Registry of Iran.33,34 Female patients aged 18 or more with HER2-negative invasive ductal BC who received NCT were considered for inclusion in this study. Patients with a prior or synchronous other malignancies and those who were lost to follow-up after surgery were excluded from the study.
The following data were obtained from the registry: age, Ki67%, grade, hormone receptor status, clinical stage, type of surgical therapy, NCT regimen, pathologic response, recurrence and survival status. Neoadjuvant regimens that were prescribed were as follows: (1) 4 cycles of doxorubicin plus cyclophosphamide (AC), (2) 4 cycles of doxorubicin plus cyclophosphamide followed by 4 cycles of paclitaxel (ACP), (3) 4 cycles of doxorubicin plus cyclophosphamide followed by 4 cycles of docetaxel (ACD), (4) 6 cycles of doxorubicin plus cyclophosphamide plus docetaxel (TAC). Response to NCT in surgical specimens were categorized into complete, partial and no response. Pathologic complete response (pCR) was defined as complete absence of viable tumor in the specimen. Pathologic partial response (pPR) and pathologic non-response (pNR) were defined as less than 50% and more than 50% of the treated tumor occupied by viable tumor cells, respectively.
Statistical Analysis
Categorical variables are presented as number (%) whereas continuous variables are shown as mean (standard deviation). Using Fisher’s exact and Chi-square tests, we assessed patient characteristics and pathologic response in relation to neoadjuvant regimens. Survival analyses were made using the Kaplan–Meier method and compared by the log-rank test. Overall survival (OS) was defined as the time from cancer diagnosis until death from any cause. Disease-free survival (DFS) was defined as the period between the date of cancer diagnosis and the date of disease recurrence or death from any cause. The Cox proportional hazards regression model was used for computing hazard ratios (HRs) and multivariate survival analysis. The cumulative incidence of recurrence rates was estimated using competing risk methods and compared by the Gray test. Estimated HRs were calculated by Fine and Gray regression modeling to evaluate effects of variables on the risk of recurrence. P values less than 0.05 were considered to be statistically significant. SPSS Statistics for Windows (version 26.0; Armonk, NY: IBM Corporation) and R software (version 4.3.1; R Core Team, Vienna, Austria) were employed for statistical analyses.
Results
Patient Characteristics
A total of 163 patients were entered into analysis. The mean age of patients at the time of diagnosis was 48.5 years. Fifteen patients (9.2%) had TNBC while the tumors of 148 patients (91.8%) were hormone-receptor positive. The characteristics of the patients are listed in Table 1. Thirty patients (18.4%) received AC regimen while 61 (37.4%), 53 (32.5%) and 19 (11.7%) patients received ACD, ACP and TAC regimens, respectively.
Table 1.
Characteristics of Patients
|
Characteristic
|
|
TAC (n=19)
|
ACP (n=53)
|
ACD (n=61)
|
AC (n=30)
|
P
Value
|
| Age |
≤ 40 |
3 (6.7%) |
14 (31.1%) |
14 (31.1%) |
14 (31.1%) |
0.14 |
| 41-50 |
10 (20.4%) |
16 (32.7%) |
17 (34.7%) |
6 (12.2%) |
| 51-60 |
4 (8.9%) |
16 (35.6%) |
21 (46.7%) |
4 (8.9%) |
| ≥ 61 |
2 (8.3%) |
7 (29.2%) |
9 (37.5%) |
6 (25%) |
| Hormone receptor |
Positive |
17 (11.5%) |
48 (32.4%) |
56 (37.8%) |
27 (18.2%) |
0.98 |
| Negative |
2 (13.3%) |
5 (33.3%) |
5 (33.3%) |
3 (20%) |
| Ki67 |
≤ 10% |
4 (9.1%) |
15 (34.1%) |
14 (31.8%) |
11 (25%) |
0.15 |
| > 10% |
14 (13.6%) |
32 (31.1%) |
45 (43.7%) |
12 (11.7%) |
| unknown |
1 (6.3%) |
6 (37.5%) |
2 (12.5%) |
7 (43.8%) |
| Grade |
1 |
2 (10%) |
3 (15%) |
10 (50%) |
5 (25%) |
0.60 |
| 2 |
12 (12.6%) |
34 (35.8%) |
34 (35.8%) |
15 (15.8%) |
| 3 |
5 (10.4%) |
16 (33.3%) |
17 (35.4%) |
10 (20.8%) |
| Clinical stage |
IIA |
5 (8.6%) |
17 (29.3%) |
21 (36.2%) |
15 (25.9%) |
0.69 |
| IIB |
6 (15%) |
10 (25%) |
18 (45%) |
6 (15%) |
| IIIA |
1 (6.7%) |
6 (40%) |
5 (33.3%) |
3 (20%) |
| IIIB |
3 (10%) |
11 (36.7%) |
12 (40%) |
4 (13.3%) |
| IIIC |
4 (20%) |
9 (45%) |
5 (25%) |
2 (10%) |
| Surgical therapy |
BCT |
5 (14.3%) |
12 (34.3%) |
12 (34.3%) |
6 (17.1%) |
0.92 |
| Mastectomy |
14 (10.9%) |
41 (32%) |
49 (38.3%) |
24 (18.8%) |
TAC, docetaxel + doxorubicin + cyclophosphamide; ACP, doxorubicin + cyclophosphamide + paclitaxel; ACD, doxorubicin + cyclophosphamide + docetaxel; AC, doxorubicin + cyclophosphamide; BCT, Breast-conserving therapy.
Response to Neoadjuvant Chemotherapy
Thirty-two patients (19.6%) experienced pCR compared with 131 patients(80.4%) who had residual disease in their surgical specimen. Significant increased pCR rates were observed for patients treated with TAC compared with another regimen (P < 0.001). In terms of pathologic response based on the neoadjuvant regimen (Table 2), patients treated with TAC showed pCR of 42.1%, pPR of 52.6%, and negligible pNR rate of 5.3%. This contrasts with the other regimens, for instance, ACP which had 18.9% pCR, 62.3% pPR, and 18.9% pNR. Patients with TNBC experienced significantly higher pCR than those with hormone receptor-positive cancer (P = 0.007). Within the subset of hormone receptor status, positive patients had pCR of 16.9%, pPR of 56.8%, and pNR of 26.4% while negative ones demonstrated pCR of 46.7%, pPR of 20%, and pNR of 33.3% (Table 2). Significantly lower pCR rates were found in patients with Ki67 ≤ 10% versus those with Ki67 > 10% (P = 0.02). Specifically, patients with Ki67 ≤ 10% exhibited pCR of 9.1%, pPR of 70.5%, and pNR of 20.5%, while those with Ki67 > 10% had pCR of 27.2%, pPR of 49.5%, and pNR of 23.3% (Table 2). Grade and clinical stage did not have any significant effect on pathologic response, although it has been observed that patients with higher stage and clinical stage tend to experience better response to NCT (Table 2).
Table 2.
Pathologic Response Based on Neoadjuvant Regimen and Patient Characteristics.
|
|
|
pCR
|
pPR
|
pNR
|
P
value
|
| Neoadjuvant regimen |
TAC |
8 (42.1%) |
10 (52.6%) |
1 (5.3%) |
< 0.001 |
| ACP |
10 (18.9%) |
33 (62.3%) |
10 (18.9%) |
| ACD |
12 (19.7%) |
39 (63.9%) |
10 (16.4%) |
| AC |
2 (6.7%) |
5 (16.7%) |
23 (76.7%) |
| Hormone receptor |
positive |
25 (16.9%) |
84 (56.8%) |
39 (26.4%) |
0.007 |
| negative |
7 (46.7%) |
3 (20%) |
5 (33.3%) |
| Ki67 |
≤ 10% |
4 (9.1%) |
31 (70.5%) |
9 (20.5%) |
0.02 |
| > 10% |
28 (27.2%) |
51 (49.5%) |
24 (23.3%) |
| Grade |
1 |
3 (15%) |
9 (45%) |
8 (40%) |
0.14 |
| 2 |
14 (14.7%) |
56 (58.9%) |
25 (26.3%) |
| 3 |
11 (22.9%) |
23 (47.9%) |
14 (29.2%) |
| Clinical Stage |
IIA |
10 (17.2%) |
26 (44.8%) |
22 (37.9%) |
0.33 |
| IIB |
8 (20%) |
26 (65%) |
6 (15%) |
| IIIA |
3 (20%) |
7 (46.7%) |
5 (33.3%) |
| IIIB |
5 (16.7%) |
18 (60%) |
7 (23.3%) |
| IIIC |
6 (30%) |
10 (50%) |
4 (20%) |
pCR, pathologic complete response; pPR, pathologic partial response; pNR, pathologic no response; TAC, docetaxel + doxorubicin + cyclophosphamide; ACP, doxorubicin + cyclophosphamide + paclitaxel; ACD, doxorubicin + cyclophosphamide + docetaxel; AC, doxorubicin + cyclophosphamide.
To underscore the distinctions between the regimens, patients treated with TAC exhibited significantly higher overall response rates, reaching 94%, compared to those on other regimens, with ACP at 79%, ACD at 88%, and AC at 80% (P < 0.001). Such stark contrasts highlight the potential superiority of TAC in eliciting favorable responses, a finding that warrants further discussion and analysis.
Survival
The OS rate at 36 months was 84.7% for all patients and was comparable among the patients who received various neoadjuvant regimen (P = 0.28, Figure 1). However, TAC results in superior 36-month OS compared with other regimens (TAC = 94%, ACP = 79%, ACD = 88%, AC = 80%). Cox proportional regression revealed that no variable significantly influences the OS (Table 3).
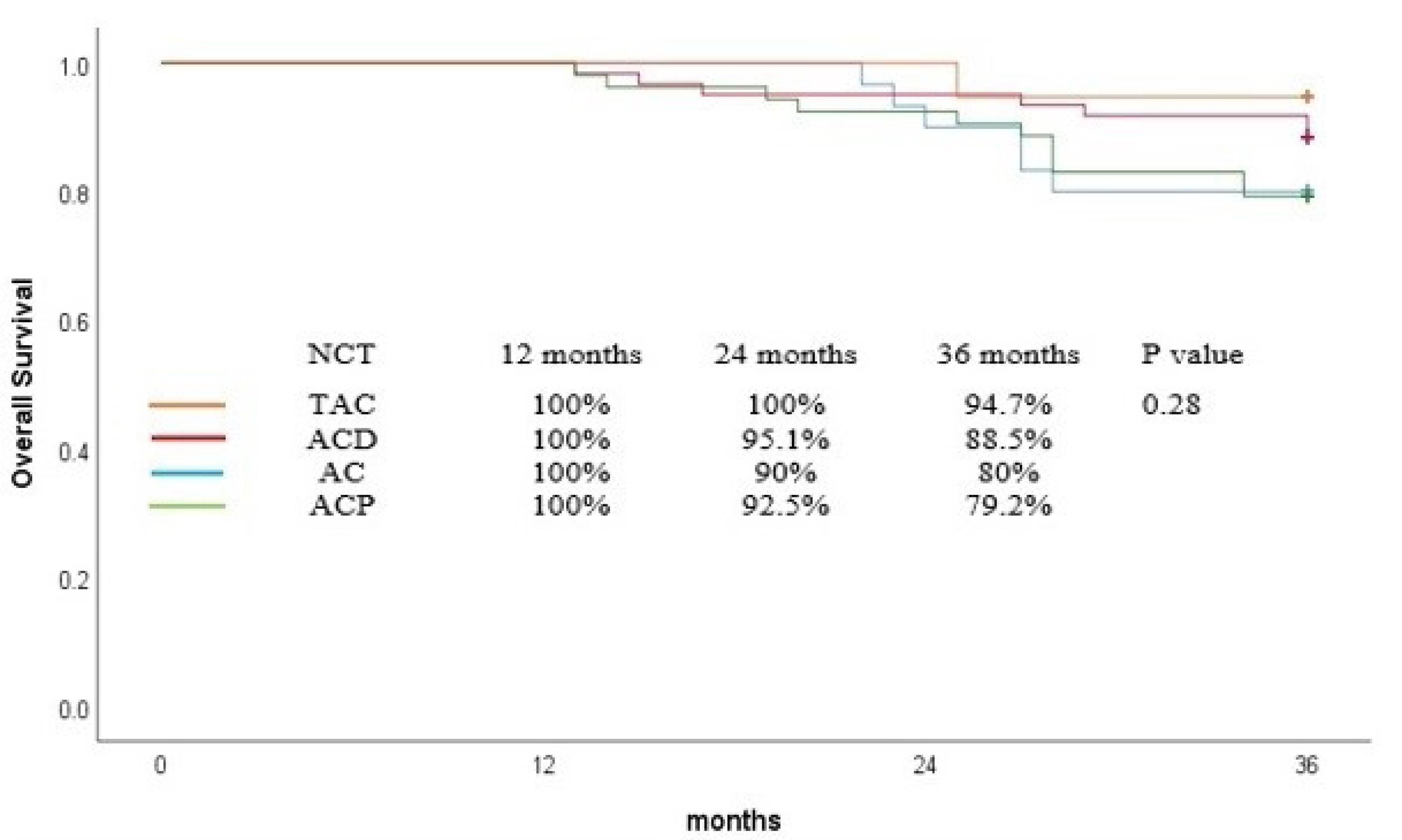
Figure 1.
Overall Survival Curve for Patients Based on Neoadjuvant Chemotherapy
.
Overall Survival Curve for Patients Based on Neoadjuvant Chemotherapy
Table 3.
Univariable Analysis for Overall Survival, Disease-free Survival and Recurrence
|
|
|
OS
|
DFS
|
Recurrence
|
|
HR (95%CI)
|
P
|
HR (95%CI)
|
P
|
HR (95%CI)
|
P
|
| Neoadjuvant regimen |
ACP vs. TAC |
4.2 (0.54-32.8) |
0.16 |
5.9 (0.78-45.1) |
0.08 |
3.32 (0.38-28.4) |
0.27 |
| ACD vs. TAC |
4.2 (0.27-18.1) |
0.45 |
3.5 (0.46-27.6) |
0.22 |
2.89 (0.36-22.8) |
0.31 |
| AC vs. TAC |
4.09 (0.49-34) |
0.19 |
4.05 (0.48-33.7) |
0.19 |
4.53 (0.59-34.9) |
0.14 |
| Age |
≤ 40 vs. ≥ 61 |
0.63 (0.19-2.07) |
0.44 |
0.93 (0.31-2.77) |
0.89 |
2.52 (0.54-11.6) |
0.23 |
| 41-50 vs. ≥ 61 |
0.69 (0.22-2.1) |
0.52 |
0.94 (0.32-2.77) |
0.92 |
2.56 (0.56-11.7) |
0.22 |
| 51-60 vs. ≥ 61 |
0.76 (0.24-2.42) |
0.65 |
0.94 (0.31-2.82) |
0.92 |
1.62 (0.32-8.04) |
0.55 |
| Hormone receptor |
Negative vs. positive |
1.35 (0.4-4.52) |
0.62 |
1.63 (0.39-6.83) |
0.5 |
1.28 (0.3-5.43) |
0.73 |
| Ki67 |
≤ 10% vs. > 10% |
0.76 (0.31-1.81) |
0.53 |
0.97 (0.44-2.11) |
0.94 |
0.86 (0.36-2.05) |
0.74 |
| Grade |
1 vs. 3 |
0.68 (0.14- 3.3) |
0.63 |
0.91 (0.24-3.45) |
0.89 |
0.81 (0.16-4.02) |
0.79 |
| 2 vs. 3 |
1.21 (0.5-2.96) |
0.66 |
1.46 (0.65-3.29) |
0.35 |
1.71 (0.68-4.28) |
0.25 |
| Clinical Stage |
IIA vs. IIIC |
3.74 (0.47-29.2) |
0.2 |
2.22 (0.49-9.91) |
0.29 |
2.22 (0.49-9.94) |
0.29 |
| IIB vs. IIIC |
3.89 (0.47-31.6) |
0.2 |
2.81 (0.61-12.8) |
0.18 |
1.88 (0.39-9.04) |
0.43 |
| IIIA vs. IIIC |
4.58 (0.47-44) |
0.18 |
2.9 (0.53-15.8) |
0.21 |
1.34 (0.18-9.51) |
0.77 |
| IIIB vs. IIIC |
2.77 (0.31-24.8) |
0.36 |
1.8 (0.35-9.29) |
0.48 |
1.39 (0.25-7.62) |
0.70 |
| Surgical therapy |
BCT vs. Mastectomy |
2.06 (0.61-6.89) |
0.23 |
1.62 (0.62-4.21) |
0.31 |
1.67 (0.57-4.83) |
0.34 |
| Pathologic response |
pCR vs. pNR |
0.22 (0.5-1.02) |
0.05 |
0.65 (0.19-2.18) |
0.49 |
0.55 (0.14-2.16) |
0.39 |
| pPR vs. pNR |
0.53 (0.23-1.2) |
0.13 |
1.35 (0.6-3.06) |
0.46 |
1.23 (0.51-2.97) |
0.64 |
OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; TAC, docetaxel + doxorubicin + cyclophosphamide; ACP, doxorubicin + cyclophosphamide + paclitaxel; ACD, doxorubicin + cyclophosphamide + docetaxel; AC, doxorubicin + cyclophosphamide; BCT, Breast-conserving therapy; pCR, pathologic complete response; pPR, pathologic partial response; pNR, pathologic no response.
Univariable Cox proportional hazards model for PFS and OS.
Univariable Fine-Gray hazards model for cumulative incidence of recurrence.
The DFS rate at 36 months was 79.8% for all patients. Similarly, TAC regimen insignificantly improved DFS in comparison with others and 36-month DFS was comparable among the patients who received different neoadjuvant regimens (TAC = 94%, ACP = 71%, ACD = 82%, AC = 80%, P = 0.19, Figure 2). In regression analyses, no variable significantly affected the DFS (Table 3).
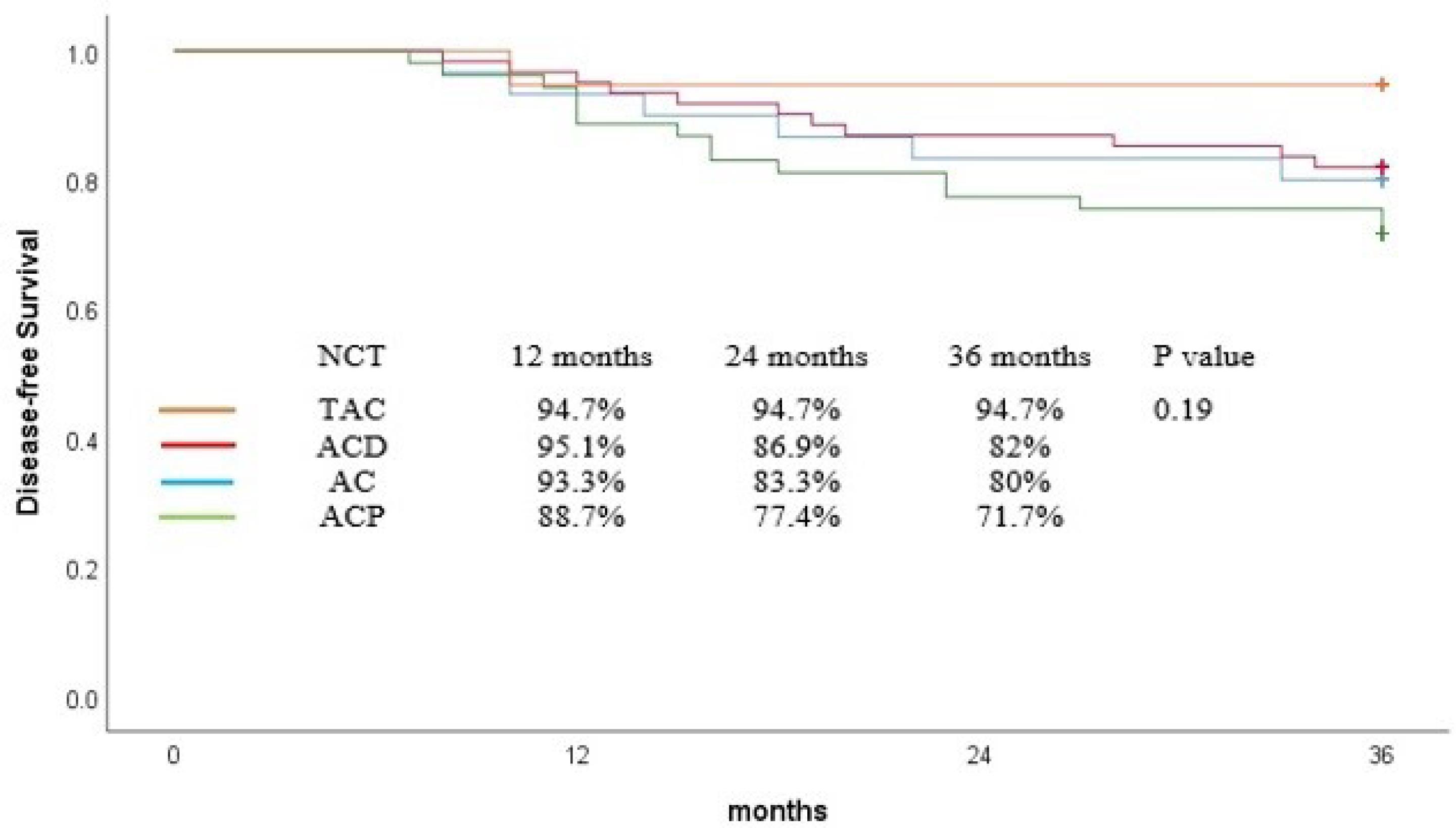
Figure 2.
Disease-Free Survival Curve for Patients Based on Neoadjuvant Chemotherapy
.
Disease-Free Survival Curve for Patients Based on Neoadjuvant Chemotherapy
Multivariable regression analysis was done to determine the potential predictors of OS and DFS. Analysis including type of neoadjuvant regimen, age, hormone receptor status, Ki67, grade, clinical stage, type of surgery and pathologic response to chemotherapy demonstrated that no variable was a statistically significant prognostic factor affecting OS and DFS (Table 4).
Table 4.
Multivariable Analysis for Overall Survival, Disease-free Survival and Recurrence
|
|
|
OS
|
DFS
|
Recurrence
|
|
HR (95% CI)
|
P
|
HR (95% CI)
|
P
|
HR (95% CI)
|
P
|
| Neoadjuvant regimen |
ACP vs. TAC |
3.49 (0.42-28.5) |
0.24 |
5.39 (0.69-41.8) |
0.10 |
4.39 (0.54-35.2) |
0.16 |
| ACD vs. TAC |
1.64 (0.19-13.7) |
0.64 |
2.97 (0.37-23.4) |
0.30 |
2.68 (0.32-22.05) |
0.35 |
| AC vs. TAC |
1.37 (0.12-15.1) |
0.19 |
4.37 (0.45-42.4) |
0.20 |
4.51 (0.45-44.63) |
0.19 |
| Age |
≤ 40 vs. ≥ 61 |
1.05 (0.28-3.97) |
0.93 |
1.10 (0.35-3.44) |
0.87 |
3.33 (0.68-16.20) |
0.13 |
| 41-50 vs. ≥ 61 |
1 (0.25-3.92) |
0.99 |
0.96 (0.30-3.09) |
0.95 |
3.02 (0.60-15.04) |
0.17 |
| 51-60 vs. ≥ 61 |
0.85 (0.21-3.50) |
0.83 |
0.79 (0.23-2.70) |
0.71 |
1.76 (0.32-9.60) |
0.51 |
| Hormone receptor |
Negative vs. positive |
1.06 (0.19-5.66) |
0.94 |
0.84 (0.18-4) |
0.83 |
1.01 (0.21-4.85) |
0.98 |
| Ki67 |
≤ 10% vs. > 10% |
0.61 (0.21-1.80) |
0.37 |
1.08 (0.45-2.62) |
0.85 |
0.71 (0.26-2.33) |
0.69 |
| Grade |
1 vs. 3 |
1.28 (0.23- 7.20) |
0.77 |
1.13 (0.28-4.56) |
0.85 |
1.04 (0.20-5.45) |
0.95 |
| 2 vs. 3 |
1.35 (0.42-4.32) |
0.60 |
1.24 (0.47-3.24) |
0.65 |
1.68 (0.59-4.73) |
0.32 |
| Clinical Stage |
IIA vs. IIIC |
2.56 (0.29-22.04) |
0.39 |
1.82 (0.39-8.43) |
0.44 |
1.57 (0.33-7.41) |
0.56 |
| IIB vs. IIIC |
4.1 (0.45-37.04) |
0.20 |
2.23 (0.46-10.6) |
0.31 |
1.29 (0.25-6.56) |
0.75 |
| IIIA vs. IIIC |
3.08 (0.22-42.65) |
0.40 |
2.13 (0.32-14.08) |
0.43 |
1.27 (0.15-10.16) |
0.82 |
| IIIB vs. IIIC |
2.55 (0.26-24.13) |
0.31 |
1.58 (0.29-8.44) |
0.59 |
1.29 (0.22-7.47) |
0.77 |
| Surgical therapy |
BCT vs. Mastectomy |
1.54 (0.41-5.72) |
0.51 |
1.34 (0.48-3.71) |
0.56 |
1.77 (0.58-5.44) |
0.31 |
| Pathologic response |
pCR vs. pNR |
0.21 (0.04-1.17) |
0.07 |
0.80 (0.19-3.39) |
0.77 |
0.53 (0.11-2.46) |
0.42 |
| pPR vs. pNR |
0.40 (0.12-1.30) |
0.13 |
1.52 (0.49-4.66) |
0.46 |
1.21 (0.39-3.70) |
0.73 |
OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; TAC, docetaxel + doxorubicin + cyclophosphamide; ACP, doxorubicin + cyclophosphamide + paclitaxel; ACD, doxorubicin + cyclophosphamide + docetaxel; AC, doxorubicin + cyclophosphamide; BCT, Breast-conserving therapy; pCR, pathologic complete response; pPR, pathologic partial response; pNR, pathologic no response.
Multivariable Cox proportional hazards model for PFS and OS.
Multivariable Fine-Gray hazards model for cumulative incidence of recurrence.
While the OS and DFS rates were generally high across all regimens, a nuanced look at the data reveals TAC’s potential edge. At 36 months, TAC achieved 94% OS and 94% DFS, outstripping ACP (79% OS, 71% DFS), ACD (88% OS, 82% DFS), and AC (80% OS, 80% DFS), although the differences were not statistically significant (OS, P = 0.28; DFS, P = 0.19). These regimen-specific outcomes illuminate the varying impacts of the treatment regimens on patient survival, underscoring the need for a tailored approach to regimen selection.
Recurrence
The recurrence rate at 36 months was 16.6% for all patients. Figure 3 shows the cumulative incidence of recurrence. There was no significant difference in the 36-month cumulative incidence rates of recurrence among patients who took different neoadjuvant regimens (TAC = 5.3%, ACP = 22.6%, ACD = 14.8%, AC = 16.7%, P = 0.38). No variable was found in univariable and multivariable analysis that significantly influenced the recurrence rate (Tables 3 and 4).
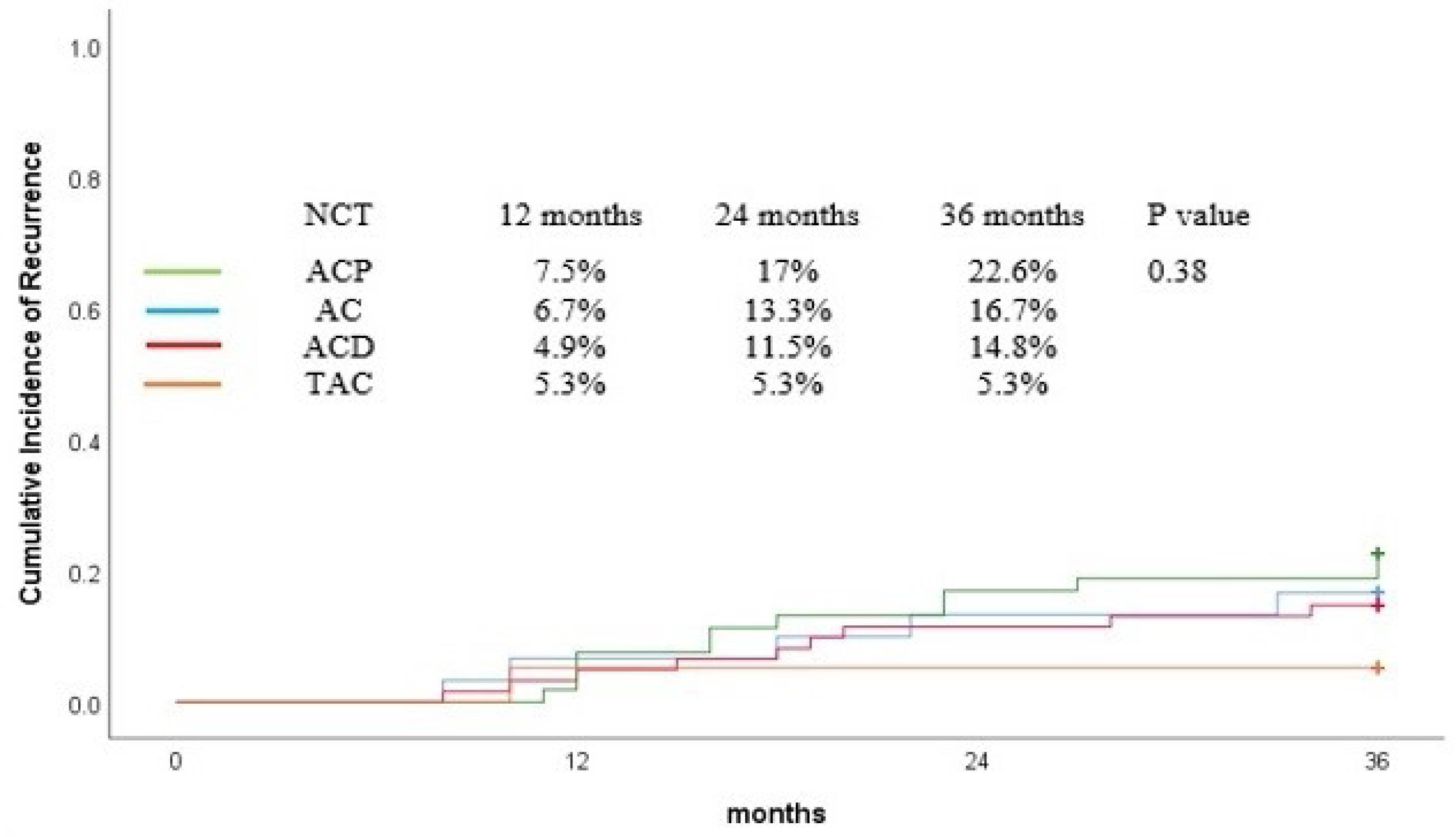
Figure 3.
Cumulative Incidence of Recurrence Curve for Patients Based on Neoadjuvant Chemotherapy
.
Cumulative Incidence of Recurrence Curve for Patients Based on Neoadjuvant Chemotherapy
Discussion
We conducted this study to evaluate the three-year survival of patients with BC who received one of four regimens of NCT and compare the outcomes of treatment among the regimens. We investigated the effect of tumor characteristics, including hormone receptor status, Ki67, grade, and clinical stage on treatment outcomes. There was no significant difference in OS and DFS rates among patients who received different NCT regimens. Meanwhile, TAC tended to result in superior OS and DFS compared with other regimens. Overall, the results showed that TAC regimen and TNBC were more associated with pCR, while patients with Ki67 ≤ 10% were less likely to achieve pCR. Although not statistically significant, patients with higher tumor grade and clinical stage seemed to exhibit a better response to NCT. These findings are consistent with previous studies that have reported the association between these factors and treatment outcomes in BC patients treated with NCT.
Delving deeper into the effects of the NCT regimens, our data suggest a promising trend for the TAC regimen, demonstrating higher pCR, OS, and DFS rates compared to other regimens. Although these differences did not reach statistical significance, the consistency of TAC’s performance across multiple outcome measures cannot be overlooked. The pronounced disparity in overall response rates, with TAC at 94% compared to 79% for ACP, 88% for ACD, and 80% for AC, points to its potential as a more efficacious option for inducing pathologic response. Similarly, the survival analysis revealed TAC’s superior performance, achieving 94% OS and 94% DFS at 36 months, a trend that held even when adjusting for various patient and tumor characteristics.
Early diagnosis and chemotherapy have improved the survival of patients with BC, although morbidities caused by chemotherapy may affect patients in the long term.35 Our investigation showed that NCT resulted in a favorable three-year OS rate of 84.7% and DFS rate of 79.8%. Most of the pCR in our study were from the TAC group. Combinations of docetaxel, doxorubicin, and cyclophosphamide have been used with favorable clinical and pathological results for treating BC. In a recent study, researchers explored the efficacy and safety of pyrotinib plus TAC for HER2 + BC.36 Overall, 37.0% of patients achieved total pCR, 37.0% pCR in the breast, and 85.2% pCR in lymph node. In a trial of TAC regimen on 45 patients with BC, authors reported a clinical response rate of 59% (95% CI 42% to 73%) within the breast and overall (breast and axilla) response rate of 49% (95% CI 38% to 72%) in the intention-to-treat population.37 The pCR was 10% in the breast, 27% in the axillary lymph nodes, and 7% in both. They also reported a 5-year survival rate of 80%. Another trial resulted in 16% pCR for a TAC group.38 Overall, TAC was a recommended regimen for BC in the literature. However, the discrepancies may be due to differences in patient populations, treatment protocols, or study designs, implying the need for further research to find the optimal NCT regimens for different patient subgroups.
A linear relationship has been suggested between increasing pCR rate and increasing recurrence-free survival.39 A meta-analysis by Cortazar et al included 11 955 patients from 12 randomized clinical trials and showed that NAC significantly improved the pCR rate and event-free survival compared with ACT.40 Meanwhile, the association between pCR and long-term outcomes was strongest in patients with aggressive tumor subtypes, such as TNBC and HER2-positive, and hormone receptor-negative tumors. In TNBC, the association between pCR and long-term outcomes was strongest with HR of 0.24 (95%CI 0.18-0.33) for event-free survival and 0.16 (0.11-0.25) for OS. Broadly speaking, TNBC is a cancer that exhibits a significant degree of heterogeneity and mutations as well as abnormal activation of signaling pathways. Recent studies suggested that targeted therapies are more promising treatment options against TNBC.6 Other studies have also reported that the attainment of a pCR after NCT in TNBC patients leads to improved survival.19,32 In a meta-analysis, Xia et al compared NCT and ACT in TNBC patients. They included nine studies with a total of 36,480 patients, where 29.41% received NCT, and 70.59% received ACT. The results showed that NCT with pCR significantly improved OS and DFS. They suggested that NCT with pCR is superior to ACT in improving survival outcomes for TNBC patients.32
Commonly, Ki67 has been proposed as a useful clinical marker for BC subtype classification, prognosis, and prediction of therapeutic response.41 A recent review study conducted by Zhang et al assessed the role of Ki67 in NCT therapy for BC.41 They concluded that NCT is the first choice for TNBC and HER2-positive BC. However, because of the uniformly low rate of pCR and slow response, NCT was not suggested as the preferred option for rapidly reducing the stage of large tumor burdens.40-42 They also reported that higher pretreatment Ki67 was more likely to attain pCR after NCT and that higher pretreatment Ki67 may improve the prognostic significance of clinical response in NCT.43-45 Our study showed that patients with TNBC experienced significantly higher pCR than those with hormone-receptor positive BC. This is consistent with a study by Colleoni et al who found a statistically significant higher pCR rate in patients with estrogen and progesterone absent tumors (adjusted OR = 14.4).46 Previous studies indicated that the absence of hormone receptor expression and Ki-67 ≥ 20% were predictive of a clinical complete response.47 A high tumor grade has been reported as a predictive factor of pCR.47 However, our results did not replicate the significant effect of tumor grade in pCR. Also, the current study did not find a significant difference in survival outcomes across patients treated with different NAC regimens. These require more delineation with a larger sample size and longer follow-up period.
One potential reason for the absence of significant differences in OS and DFS across the different NCT regimens may be attributed to the inherent variability in patient and tumor characteristics, which have a profound influence on treatment outcomes. It is conceivable that while the chemotherapeutic agents themselves possess distinct mechanisms of action, the nuances in individual patient profiles, tumor biology, and disease staging could counterbalance these differences, leading to analogous survival rates. Furthermore, the intricacies of tumor microenvironment interactions, resistance mechanisms, and other yet unidentified biological variables might play a pivotal role in determining individual responses to treatment, thus nullifying any overt differences among the regimens. While our study did illuminate certain trends, such as TAC’s superior performance in terms of pCR, OS, and DFS rates, it is crucial to highlight the possible presence of confounding variables or biases that might have been inadvertently introduced during patient selection, treatment assignment, or data interpretation. A more comprehensive, prospective study with stratified randomization and longer follow-up might offer deeper insight into the precise reasons behind the observed equivalence in survival rates among the NCT regimens.
In the groundbreaking IMpassion130 trial led by Schmid et al, the combined therapeutic efficacy of Atezolizumab and Nab-Paclitaxel was assessed in patients with metastatic TNBC. With their patient cohort having a median age of 55 years in comparison to the average age of 48.5 years in our study, the IMpassion130 trial reported an objective response rate of 53%, standing in contrast to our pCR rate of 19.6%. Furthermore, the IMpassion130 trial noted a median progression-free survival (PFS) of 7.2 months. This, when compared with our more extended DFS rate of 79.8% at 36 months and an OS rate of 84.7%, underscores the potential disparities in treatment outcomes based on therapeutic choices and patient cohorts.48
In a study conducted by Yamamoto et al49 researchers explored the potential advantages of adjuvant capecitabine for HER2-negative BC patients who had residual disease following neoadjuvant treatment. The study’s participants presented a 5-year OS rate of 89.2%, aligning closely yet slightly exceeding our 36-month OS rate of 84.7%. Their DFS, reported at 74.1% over 5 years, also mirrors our 36-month statistic of 79.8%. Although the direct response rate in terms of pCR for the CREATE-X trial is not directly comparable, the similarities and contrasts in survival metrics between our research and theirs highlight the pivotal role of tailored therapeutic strategies, and the significance of deep-diving into specific patient and tumor characteristics in driving optimal care decisions.
In the renowned NSABP B-27 trial orchestrated by the National Surgical Adjuvant Breast and Bowel Project, the potential of introducing docetaxel to a foundational regimen of AC in the neoadjuvant setting was closely examined for operable BC, inclusive of the HER2-negative subtype.50 Participants, who were subjected to varying sequences of the aforementioned drugs, presented a pCR rate of 26.1% when doxorubicin and cyclophosphamide were succeeded by docetaxel. This stands in contrast with our cohort, composed of patients with an average age of 48.5 years, which manifested a pCR rate of 19.6%. While the NSABP B-27 trial did not note pronounced disparities in OS between their arms at the five-year benchmark, our study highlighted an OS rate of 84.7% at 36 months. Similarly, the trial’s modest enhancement in DFS with the inclusion of docetaxel resonates with our compelling DFS rate of 79.8% over the same 36-month span. The juxtaposition of these investigations accentuates the intricate tapestry of neoadjuvant therapeutic strategies and underscores the import of regimen sequencing and drug combination in achieving optimal patient outcomes.
In a significant undertaking by Kim et al, researchers embarked on contrasting the outcomes of neoadjuvant endocrine therapy (NET) with those of NCT in pre-menopausal patients diagnosed with ER-positive, HER2-negative, lymph node-positive BC.51 Sourced from seven hospitals in South Korea, the patients in their study, who underwent 24 weeks of either therapeutic regimen, painted a decisive picture: those under NCT displayed a markedly higher clinical response rate of 83.7% versus the 52.9% in the NET cohort. This discrepancy is pronounced when juxtaposed with our study, where participants (with an average age of 48.5 years) reported a pCR rate of 19.6%. Kim and colleagues also observed a marginally higher pCR in the NCT group (3.4%) compared to the NET group (1.2%). In comparison, our study flaunted a robust OS of 84.7% at 36 months and a DFS rate of 79.8%. The differential outcomes between the investigation by Kim et al and ours accentuate the therapeutic efficacy of individualized treatments, emphasizing the ever-evolving dynamics of BC care.
In general, our findings are consistent with previous studies that have reported improved survival outcomes with NCT in patients with BC. For instance, in a trial of NCT for 72 BCs, mastectomy was avoided in 46% of patients, 42% converted to negative nodes after NCT, and 18% achieved a pCR.13 Five-year survival for patients with pCR was 100%, compared with 74% in the group with partial response and 48% in the group with no response or progression. Patients with the ER + /HER2 + subtype were most likely to have no response or progression during chemotherapy. Five-year survival was highest for patients achieving pCR. They concluded that NCT decreased the mastectomy rate, and reduced the need for axillary lymph node dissection. In a systematic review of 14 randomized trials with 5500 women, Mieog et al assessed the effectiveness of NCT versus ACT for early BC.29 They found that OS was equivalent in both groups, but the neoadjuvant group had fewer adverse effects. There was no survival difference between NCT and ACT (HR = 0.98, 95% CI = 0.87 to 1.09). It was concluded that NCT is an established treatment option for early BC. In a meta-analysis, Chen et al compared the survival benefits of NCT versus ACT for operable BC.24 The study reviewed 16 randomized clinical trials. Overall, 787 deaths were reported among 2794 patients assigned to NCT groups and 816 deaths among 2799 patients assigned to ACT groups. Subgroup analysis indicated that patients with pCR had better survival outcomes. The authors concluded that there was no significant difference in OS or recurrence-free survival between NCT and ACT groups.
The relationship between treatment response and survival outcomes, particularly OS and DFS, has been a point of interest in oncological research. It is widely understood that achieving pCR often correlates with improved survival rates. In our study, while there was no significant difference in OS and DFS rates among different NCT regimens, a noticeable trend was observed where TAC exhibited higher pCR, OS, and DFS. This suggests a potential association between pCR achievement and favorable long-term outcomes. Specifically, the pronounced pCR rates achieved by the TAC regimen may be indicative of its superior ability to eradicate micrometastatic disease, thereby leading to improved OS and DFS rates. Furthermore, the trend of higher tumor grade and clinical stage of patients exhibiting a better response to NCT underscores the potential of these tumor characteristics to predict response to therapy. A positive treatment response not only suggests a reduction in the primary tumor but may also represent an effective systemic control, which subsequently leads to enhanced OS and DFS. However, it is essential to recognize that while pCR is a strong surrogate marker, other factors, including tumor biology and individual patient characteristics, play a pivotal role in long-term survival. Future studies should further explore this relationship to optimize treatment strategies based on individual patient profiles.
One limitation of this study is its retrospective design, which may have led to selection bias. In addition, the follow-up period of three years may not have been sufficient to evaluate the long-term survival outcomes of patients with BC treated with NCT. Future prospective longitudinal studies with larger sample sizes and longer follow-up periods are needed to establish our findings and identify patient subgroups that may benefit the most from NCT.
Conclusion
In conclusion, this study provides further evidence that NCT is a viable treatment option for patients with BC, with favorable survival outcomes at least for three years. In summary, this study showed that that TAC regimen and TNBC were more associated with pCR, while patients with Ki67 ≤ 10% were less likely to achieve pCR. The TAC regimen resulted in a significantly higher pCR rate compared to other regimens, but did not result in a significant difference in recurrence, OS and DFS rates.
Acknowledgements
Authors would like to appreciate the support and constructive comments of the methodologist research development office, Imam Khomeini Hospital complex, Tehran, Iran.
Competing Interests
The authors declare no conflict of interest.
Ethical Approval
The study was approved by the ethical committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.IKHC.REC.1400.241). This study is conducted on recorded data of the Clinical Breast Cancer Registry of Iran, and all the performed procedures being performed were done according to the routine policy of medical institutions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev 2016; 17(S3):43-6. doi: 10.7314/apjcp.2016.17.s3.43 [Crossref] [ Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359-86. doi: 10.1002/ijc.29210 [Crossref] [ Google Scholar]
- Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy?. Nat Rev Clin Oncol 2009; 6(12):718-30. doi: 10.1038/nrclinonc.2009.166 [Crossref] [ Google Scholar]
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019; 321(3):288-300. doi: 10.1001/jama.2018.19323 [Crossref] [ Google Scholar]
- Gonzalez L, Bardach A, Palacios A, Peckaitis C, Ciapponi A, Pichón-Riviere A. Health-related quality of life in patients with breast cancer in Latin America and the Caribbean: a systematic review and meta-analysis. Oncologist 2021; 26(5):e794-806. doi: 10.1002/onco.13709 [Crossref] [ Google Scholar]
- Li Y, Zhang H, Merkher Y, Chen L, Liu N, Leonov S. Recent advances in therapeutic strategies for triple-negative breast cancer. J Hematol Oncol 2022; 15(1):121. doi: 10.1186/s13045-022-01341-0 [Crossref] [ Google Scholar]
- Masoud V, Pagès G. Targeted therapies in breast cancer: new challenges to fight against resistance. World J Clin Oncol 2017; 8(2):120-34. doi: 10.5306/wjco.v8.i2.120 [Crossref] [ Google Scholar]
- Gadi V, Gupta D, Shetty S. Emerging potentials of nanotherapeutics in breast cancer microenvironment targeting. OpenNano 2022; 8:100101. doi: 10.1016/j.onano.2022.100101 [Crossref] [ Google Scholar]
- Kerr AJ, Dodwell D, McGale P, Holt F, Duane F, Mannu G. Adjuvant and neoadjuvant breast cancer treatments: a systematic review of their effects on mortality. Cancer Treat Rev 2022; 105:102375. doi: 10.1016/j.ctrv.2022.102375 [Crossref] [ Google Scholar]
- Pathak M, Dwivedi SN, Deo SV, Thakur B, Sreenivas V, Rath GK. Neoadjuvant chemotherapy regimens in treatment of breast cancer: a systematic review and network meta-analysis protocol. Syst Rev 2018; 7(1):89. doi: 10.1186/s13643-018-0754-1 [Crossref] [ Google Scholar]
- Untch M, Konecny GE, Paepke S, von Minckwitz G. Current and future role of neoadjuvant therapy for breast cancer. Breast 2014; 23(5):526-37. doi: 10.1016/j.breast.2014.06.004 [Crossref] [ Google Scholar]
- Atzori G, Gipponi M, Cornacchia C, Diaz R, Sparavigna M, Gallo M. “No Ink on Tumor” in breast-conserving surgery after neoadjuvant chemotherapy. J Pers Med 2022; 12(7):1031. doi: 10.3390/jpm12071031 [Crossref] [ Google Scholar]
- Spanheimer PM, Carr JC, Thomas A, Sugg SL, Scott-Conner CE, Liao J. The response to neoadjuvant chemotherapy predicts clinical outcome and increases breast conservation in advanced breast cancer. Am J Surg 2013; 206(1):2-7. doi: 10.1016/j.amjsurg.2012.10.025 [Crossref] [ Google Scholar]
- Franceschini G, Di Leone A, Natale M, Sanchez MA, Masett R. Conservative surgery after neoadjuvant chemotherapy in patients with operable breast cancer. Ann Ital Chir 2018; 89:290. [ Google Scholar]
- Urueña C, Lasso P, Bernal-Estevez D, Rubio D, Salazar AJ, Olaya M. The breast cancer immune microenvironment is modified by neoadjuvant chemotherapy. Sci Rep 2022; 12(1):7981. doi: 10.1038/s41598-022-12108-5 [Crossref] [ Google Scholar]
- Masood S. Neoadjuvant chemotherapy in breast cancers. Womens Health (Lond) 2016; 12(5):480-91. doi: 10.1177/1745505716677139 [Crossref] [ Google Scholar]
- Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376(22):2147-59. doi: 10.1056/NEJMoa1612645 [Crossref] [ Google Scholar]
- Tian H, Ma D, Tan X, Yan W, Wu X, He C. Platinum and taxane based adjuvant and neoadjuvant chemotherapy in early triple-negative breast cancer: a narrative review. Front Pharmacol 2021; 12:770663. doi: 10.3389/fphar.2021.770663 [Crossref] [ Google Scholar]
- Li ZY, Zhang Z, Cao XZ, Feng Y, Ren SS. Platinum-based neoadjuvant chemotherapy for triple-negative breast cancer: a systematic review and meta-analysis. J Int Med Res 2020; 48(10):0300060520964340. doi: 10.1177/0300060520964340 [Crossref] [ Google Scholar]
- McGuirk S, Audet-Delage Y, Annis MG, Xue Y, Vernier M, Zhao K. Resistance to different anthracycline chemotherapeutics elicits distinct and actionable primary metabolic dependencies in breast cancer. Elife 2021; 10:e65150. doi: 10.7554/eLife.65150 [Crossref] [ Google Scholar]
- Abu Samaan TM, Samec M, Liskova A, Kubatka P, Büsselberg D. Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules 2019; 9(12):789. doi: 10.3390/biom9120789 [Crossref] [ Google Scholar]
- Hong K, Yao L, Sheng X, Ye D, Guo Y. Neoadjuvant therapy of cyclin-dependent kinase 4/6 inhibitors combined with endocrine therapy in HR + /HER2- breast cancer: a systematic review and meta-analysis. Oncol Res Treat 2021; 44(10):557-67. doi: 10.1159/000518573 [Crossref] [ Google Scholar]
- Derouane F, van Marcke C, Berlière M, Gerday A, Fellah L, Leconte I. Predictive biomarkers of response to neoadjuvant chemotherapy in breast cancer: current and future perspectives for precision medicine. Cancers (Basel) 2022; 14(16):3876. doi: 10.3390/cancers14163876 [Crossref] [ Google Scholar]
- Chen Y, Shi XE, Tian JH, Yang XJ, Wang YF, Yang KH. Survival benefit of neoadjuvant chemotherapy for resectable breast cancer: a meta-analysis. Medicine (Baltimore) 2018; 97(20):e10634. doi: 10.1097/md.0000000000010634 [Crossref] [ Google Scholar]
- Fitzpatrick A, Tutt A. Controversial issues in the neoadjuvant treatment of triple-negative breast cancer. Ther Adv Med Oncol 2019; 11:1758835919882581. doi: 10.1177/1758835919882581 [Crossref] [ Google Scholar]
- Fukada I, Araki K, Kobayashi K, Shibayama T, Takahashi S, Gomi N. Pattern of tumor shrinkage during neoadjuvant chemotherapy is associated with prognosis in low-grade luminal early breast cancer. Radiology 2018; 286(1):49-57. doi: 10.1148/radiol.2017161548 [Crossref] [ Google Scholar]
- Huang Y, Chen W, Zhang X, He S, Shao N, Shi H. Prediction of tumor shrinkage pattern to neoadjuvant chemotherapy using a multiparametric MRI-based machine learning model in patients with breast cancer. Front Bioeng Biotechnol 2021; 9:662749. doi: 10.3389/fbioe.2021.662749 [Crossref] [ Google Scholar]
- Liu CH, Yang JR, Tsai IC, Hsu CY, Yean LC, Hung CC. Comparison of overall survival after neoadjuvant and adjuvant chemotherapy in patients with early breast cancer with immediate breast reconstruction after mastectomy: a retrospective, matched case-control study. Oncol Lett 2022; 24(6):437. doi: 10.3892/ol.2022.13557 [Crossref] [ Google Scholar]
- Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 2007; 94(10):1189-200. doi: 10.1002/bjs.5894 [Crossref] [ Google Scholar]
- Taucher S, Steger GG, Jakesz R, Tausch C, Wette V, Schippinger W. The potential risk of neoadjuvant chemotherapy in breast cancer patients--results from a prospective randomized trial of the Austrian Breast and Colorectal Cancer Study Group (ABCSG-07). Breast Cancer Res Treat 2008; 112(2):309-16. doi: 10.1007/s10549-007-9844-9 [Crossref] [ Google Scholar]
- Gianni L, Baselga J, Eiermann W, Porta VG, Semiglazov V, Lluch A. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol 2009; 27(15):2474-81. doi: 10.1200/jco.2008.19.2567 [Crossref] [ Google Scholar]
- Xia LY, Hu QL, Zhang J, Xu WY, Li XS. Survival outcomes of neoadjuvant versus adjuvant chemotherapy in triple-negative breast cancer: a meta-analysis of 36,480 cases. World J Surg Oncol 2020; 18(1):129. doi: 10.1186/s12957-020-01907-7 [Crossref] [ Google Scholar]
- Seyyedsalehi MS, Nahvijou A, Haghjooy Javanmard S, Vand Rajabpour M, Manteghinejad A, Pirnejad H. Clinical breast cancer registry of IR Iran (CBCR-IR): study protocol and first Results. Arch Iran Med 2023; 26(11):607-17. doi: 10.34172/aim.2023.90 [Crossref] [ Google Scholar]
- Seyyedsalehi MS, Nahvijou A, Rouhollahi M, Teymouri F, Mirjomehri L, Zendehdel K. Clinical cancer registry of the Islamic Republic of Iran: steps for establishment and results of the pilot phase. J Registry Manag 2020; 47(4):200-6. [ Google Scholar]
- Haghazali M, Habibi Khorasani S, Alizadehasl A, Biglarian A, Mousavi SA, Noohi F. Echocardiographic follow-up in HER2 positive and negative breast cancer patients: is there a sustained decline in left ventricular function parameters following chemotherapy?. Int Cardiovasc Res J 2021; 15(3):e117816. [ Google Scholar]
- Tian C, Wang M, Liu H, Liu J, Xu M, Ma L. Efficacy and safety of neoadjuvant pyrotinib plus docetaxel/liposomal doxorubicin/cyclophosphamide for HER2-positive breast cancer. Ir J Med Sci 2023; 192(3):1041-9. doi: 10.1007/s11845-022-03093-9 [Crossref] [ Google Scholar]
- Gradishar WJ, Wedam SB, Jahanzeb M, Erban J, Limentani SA, Tsai KT. Neoadjuvant docetaxel followed by adjuvant doxorubicin and cyclophosphamide in patients with stage III breast cancer. Ann Oncol 2005; 16(8):1297-304. doi: 10.1093/annonc/mdi254 [Crossref] [ Google Scholar]
- Vriens BE, Aarts MJ, de Vries B, van Gastel SM, Wals J, Smilde TJ. Doxorubicin/cyclophosphamide with concurrent versus sequential docetaxel as neoadjuvant treatment in patients with breast cancer. Eur J Cancer 2013; 49(15):3102-10. doi: 10.1016/j.ejca.2013.06.012 [Crossref] [ Google Scholar]
- Hatzis C, Symmans WF, Zhang Y, Gould RE, Moulder SL, Hunt KK. Relationship between complete pathologic response to neoadjuvant chemotherapy and survival in triple-negative breast cancer. Clin Cancer Res 2016; 22(1):26-33. doi: 10.1158/1078-0432.ccr-14-3304 [Crossref] [ Google Scholar]
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384(9938):164-72. doi: 10.1016/s0140-6736(13)62422-8 [Crossref] [ Google Scholar]
- Zhang A, Wang X, Fan C, Mao X. The role of Ki67 in evaluating neoadjuvant endocrine therapy of hormone receptor-positive breast cancer. Front Endocrinol (Lausanne) 2021; 12:687244. doi: 10.3389/fendo.2021.687244 [Crossref] [ Google Scholar]
- Barchiesi G, Mazzotta M, Krasniqi E, Pizzuti L, Marinelli D, Capomolla E. Neoadjuvant endocrine therapy in breast cancer: current knowledge and future perspectives. Int J Mol Sci 2020; 21(10):3528. doi: 10.3390/ijms21103528 [Crossref] [ Google Scholar]
- Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 2005; 23(28):7212-20. doi: 10.1200/jco.2005.07.501 [Crossref] [ Google Scholar]
- Faneyte IF, Schrama JG, Peterse JL, Remijnse PL, Rodenhuis S, van de Vijver MJ. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer 2003; 88(3):406-12. doi: 10.1038/sj.bjc.6600749 [Crossref] [ Google Scholar]
- Chen R, Ye Y, Yang C, Peng Y, Zong B, Qu F. Assessment of the predictive role of pretreatment Ki-67 and Ki-67 changes in breast cancer patients receiving neoadjuvant chemotherapy according to the molecular classification: a retrospective study of 1010 patients. Breast Cancer Res Treat 2018; 170(1):35-43. doi: 10.1007/s10549-018-4730-1 [Crossref] [ Google Scholar]
- Colleoni M, Bagnardi V, Rotmensz N, Gelber RD, Viale G, Pruneri G. Increasing steroid hormone receptors expression defines breast cancer subtypes non responsive to preoperative chemotherapy. Breast Cancer Res Treat 2009; 116(2):359-69. doi: 10.1007/s10549-008-0223-y [Crossref] [ Google Scholar]
- Petit T, Wilt M, Velten M, Millon R, Rodier JF, Borel C. Comparative value of tumour grade, hormonal receptors, Ki-67, HER-2 and topoisomerase II alpha status as predictive markers in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy. Eur J Cancer 2004; 40(2):205-11. doi: 10.1016/s0959-8049(03)00675-0 [Crossref] [ Google Scholar]
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018; 379(22):2108-21. doi: 10.1056/NEJMoa1809615 [Crossref] [ Google Scholar]
- Yamamoto D, Sato N, Rai Y, Yamamoto Y, Saito M, Iwata H. Efficacy and safety of low-dose capecitabine plus docetaxel versus single-agent docetaxel in patients with anthracycline-pretreated HER2-negative metastatic breast cancer: results from the randomized phase III JO21095 trial. Breast Cancer Res Treat 2017; 161(3):473-82. doi: 10.1007/s10549-016-4075-6 [Crossref] [ Google Scholar]
- Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2003; 21(22):4165-74. doi: 10.1200/jco.2003.12.005 [Crossref] [ Google Scholar]
- Kim HJ, Noh WC, Lee ES, Jung YS, Kim LS, Han W. Efficacy of neoadjuvant endocrine therapy compared with neoadjuvant chemotherapy in pre-menopausal patients with oestrogen receptor-positive and HER2-negative, lymph node-positive breast cancer. Breast Cancer Res 2020; 22(1):54. doi: 10.1186/s13058-020-01288-5 [Crossref] [ Google Scholar]