Arch Iran Med. 26(8):447-454.
doi: 10.34172/aim.2023.68
Review
Emerging Role of Tumor-Educated Platelets as a New Liquid Biopsy Tool for Colorectal Cancer
Hossein Razzaghi Writing – original draft, 1 
Milad Khabbazpour Writing – original draft, 2
Zohreh Heidary Methodology, Writing – original draft, 3
Mohammad Heiat Supervision, Writing – review & editing, 4
Zeinab Shirzad Moghaddam Writing – original draft, 5
Parisa Derogar Writing – original draft, Writing – review & editing, 2
Ahmad Khoncheh Writing – review & editing, 4
Majid Zaki-Dizaji Conceptualization, Methodology, Supervision, Writing – review & editing, 2, * 
Author information:
1Department of Laboratory Sciences, Faculty of Paramedicine, AJA University of Medical Sciences, Tehran, Iran
2Human Genetics Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
3Vali-e-Asr Reproductive Health Research Center, Family Health Research Institute, Tehran University of Medical Sciences, Tehran, Iran
4Baqiyatallah Research Center for Gastroenterology and Liver Diseases (BRCGL), Clinical Sciences Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran
5Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Colorectal cancer (CRC) is a major cause of cancer-associated death universally. Currently, the diagnosis, prognosis, and treatment monitoring of CRC mostly depends on endoscopy integrated with tissue biopsy. Recently, liquid biopsy has gained more and more attention in the area of molecular detection and monitoring of tumors due to ease of sampling, and its safe, non-invasive, and dynamic nature. Platelets, despite their role in hemostasis and thrombosis, are known to have an active, bifacial relationship with cancers. Platelets are the second most common type of cell in the blood and are one of the wealthy liquid biopsy biosources. These cells have the potential to absorb nucleic acids and proteins and modify their transcriptome with regard to external signals, which are termed tumor-educated platelets (TEPs). Liquid biopsies depend on TEPs’ biomarkers which can be used to screen and also detect cancer in terms of prognosis, personalized treatment, monitoring, and prediction of recurrence. The value of TEPs as an origin of tumor biomarkers is relatively new, but platelets are commonly isolated using formidable and rapid techniques in clinical practice. Numerous preclinical researches have emphasized the potential of platelets as a new liquid biopsy biosource for detecting several types of tumors. This review discusses the potential use of platelets as a liquid biopsy for CRC.
Keywords: Colorectal cancer, Tumor-educated platelets, Diagnosis, Liquid biopsy, Biomarker
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Razzaghi H, Khabbazpour M, Heidary Z, Heiat M, Shirzad Moghaddam Z, Derogar P, et al. Emerging role of tumor-educated platelets as a new liquid biopsy tool for colorectal cancer. Arch Iran Med. 2023;26(8):447-454. doi: 10.34172/ aim.2023.68
Introduction
Colorectal cancer (CRC) is the second deadliest cancer and the third most common cancer globally.1,2 During 2018, there were 1.8 million new patients of CRC and 881 000 deaths worldwide, which accounted for close to 10% of all new cancer cases and deaths.1 Unfortunately, 20% of newly diagnosed CRC cases are metastatic, and 25% of those with the localized disease will later develop metastases.3 The incidence and mortality rates are rising annually, and the growth of the population and the aging of society may further increase this incidence, posing a grave threat to public health.
Up to now, the screening and detection of CRC generally depend on the assessment of serum biomarkers, tissue biopsy, and imaging.4 Nevertheless, recent studies demonstrated that the accuracy and sensitivity of imaging and pathological approaches are still restricted, and the specificity of the serum biomarkers is low. Also, as a major problem, it is challenging to perform repeated biopsies for early cancer detection, monitoring of disease progression, and further observation of tumor resistance mutations.5 Furthermore, a one-only biopsy typically does not represent a patient’s tumoral heterogeneity.1 Given that the survival of patients with CRC is extremely dependent on early detection and treatment, suitable biomarkers that can detect CRC and predict the therapy response in a timely fashion are urgently required.6
The primary tumor tissue does not every time deliver sufficient evidence to distinguish individual patients for the purpose of maximally efficient treatment; so, it is imperative to develop a minimally invasive technique to screen high-risk people and identify the presence of CRC in asymptomatic cases at an early and treatable stage.1,7
Liquid Biopsy
Lack of early diagnostic methods and drug resistance are the most critical factors for the high mortality rate of cancer by indirectly inducing distant metastasis.8 With the fast advancement of isolation and gene identification technologies, the central role of liquid biopsy in tumor precision medicine has become ever more apparent.9 Liquid biopsies are samples involving blood, stool, urine, pleural fluid, saliva, and cerebrospinal fluid (CSF) collected for various analyses.9,10 Compared to tissue biopsy, liquid biopsy has advantages including easy accessibility and may cover the Mutational heterogeneity between cancer cells, whereas tissue biopsy is restricted to the changes in the tumor samples. Components of blood could supply a comprehensive real-time portrait of the tumor-connected alterations, which could be used for cancer screening, early diagnosis, and surveillance of dynamic variations in the molecular level of the tumor and resistance mechanism, which could powerfully aid oncologists in guidance of treatment and monitoring of patient’s response to treatment.11 Liquid biopsy as a new technique can be used to detect evidence of tumor secreted from a primary tumor and metastasis sites by investigating tumor-related molecular markers through circulating tumor cells (CTCs), circulating free (cf) DNA or RNA, circulating tumor DNA (ctDNA), tumor-educated platelets (TEPs), extracellular vesicles (EVs) such as exosomes, and circulating tumor-derived endothelial cells (CTECs).9,12-14 Currently, CTCs, ctDNA, exosomes, and TEPs have attracted most of the attention in the liquid biopsy research (Table 1).
Table 1.
Characteristics of Liquid Biopsies
|
Liquid Biopsy Component
|
Advantage
|
Limitation
|
Half-life
|
| CTCs |
tumor cells allow for morphologic and molecular characterization of tumor, CTCs are live cells that can be used for drug screens and other functional assays |
Scarcity (1-100 CTC per mL of blood), short half-life, physical and phenotypic diversity of the marker, difficulty in isolation, undetectable in many patients at early stage |
1–2.5 h |
| ctDNA |
Well-validated, reproducible
technologies, mutation and epigenetic analysis of tumors |
Only information from apoptotic or dead tumor cells, short half-life, undetectable in many patients at early stage |
16 min |
| EVs |
Exist in almost all body fluids, high concentration (~109 particles/mL) in biofluids, high stability, secretion from living parental cells |
Lack of high-quality isolation and component analysis devices, limited sensitivity, specificity, and low purity of current isolation methods |
Several minutes to hours |
| TEPs |
Easy access and isolation, abundance of RNA, long half-life |
Lack of sufficient evidence |
7-10 days |
CTC, circulating tumor cell; ctDNA, circulating tumor DNA; EV, extracellular vesicle; TEP, tumor-educated platelet.
CTCs are cells secreted from a primary and metastatic tumor into the circulatory system that can prepare valuable tumor-related information at the molecular level, including the DNA, RNA and protein. CTC testing in cancer patients as a real-time “liquid biopsy” can aid in tumor discovery, treatment monitoring, prognosis and personalized treatment.15 CTCs could independently predict survival in CRC, after adjusting for clinically important factors including the stage of cancer.7,16 CTCs are very rare because their half-life in the bloodstream is only 1.2 to 2.4 hours, and the majority of these cells are destroyed in the circulation because of physical and environmental damage such as shear stress, immune system attacks, and oxidative stress.16-18 Although CTC research may enable thorough investigations at the molecular level (RNA, DNA, and protein levels), their variability and paucity cause challenges in gaining a thorough knowledge of these cells.19 Generally, the small number of CTCs (~1-10) in the blood restricts their use and further technological improvement. However, whole genome sequencing and whole exome sequencing of CTCs ameliorate this restriction through unraveling the cancer heterogeneity.9 CTC-based cancer monitoring by the accounting of CTCs in the whole blood, CELLSEARCH® System, was introduced to the clinic in cancer patients with metastatic breast, colorectal or prostate cancer. The presence of CTC detected by CELLSEARCH® System is correlated with decreased overall survival and progression-free survival in patients.20 Building experimental models, in vitro and in vivo, using CTCs can provide a wealth of genetic and epigenetic data on the molecular biology of tumors and allow for the evaluation of sensitivity to anticancer medicines.21
Circulating cell-free DNA (ccfDNA) are single and double-stranded DNA fragments ( ≤ 200 pb) secreted into the body fluids by apoptotic/necrotic cells. The ccfDNA secreted by cancer cells is called ctDNA and consists of a generally small fraction of the total ccfDNA in the blood.22 Since ctDNA cannot yet be differentiated from other circulating DNAs, its presence can only be determined by looking for tumor-specific mutations in cfDNA.23 Following blood collection, DNA released from dying blood cells taints and dilutes ctDNA. The main sources of cfDNA in healthy persons include the hematological system, the gastrointestinal tract, and the skin, but mostly white blood cells.24 CRC patients show greater levels of cfDNA than healthy people.25,26 In general, cfDNA examination can be used to detect point mutations or structural changes, microsatellite alterations, copy-number variations, cfDNA length variations, and methylation profile.27 Both CTCs and ctDNA are now used in standard clinical practice. CTCs testing allows for morphologic and molecular characterization of the tumor, while ctDNA examination is currently restricted to mutation discovery and epigenetic analysis.28 In CRC, ctDNA is a valuable liquid biopsy because it conveys numerous genetic changes that have similar genetic alterations as their origin tissue. Now, several ctDNA-based methods have been added to CRC screening, diagnosis and prognosis methods.15
Exosomes (40–150 nm in diameter), a subset of EVs, are a heterogeneous class of vesicles with membrane that are actively secreted through a variety of cells and stably circulate in body liquids. They may pass the blood–brain barrier (BBB) and are found in various physiological fluids, including the CSF, plasma, urine, milk, amniotic fluid, and saliva.29 Exosomes, as novel tools of cell-cell communication, encompass a various spectrum of biologically active molecules, including nucleic acids (DNA, mRNA, lncRNA, miRNA, etc.), proteins and lipids, which mirror the composition of their originating cells.30 Exosomes can be found in nearly all body fluids, have high stability owing to the lipid bilayer, and transfer biological contents from the living parental cells.31 The exosomes derived from cancers are related to tumor growth, metastatic niches development, and evasion from the immune system.32 The diagnostic and prognostic potentials of exosomal biomarkers have been studied in several cancers. However, these cancer-related exosomes are just a minor portion of all exosomes existing in body fluids; therefore, highly sensitive detection methods are needed to isolate exosomes of interest with high efficiency and purity, in nanoscale size and intrinsic heterogeneity of exosomes.31 Currently, Exosome Diagnostics has introduced a few exosome-based tools for some cancers, including ExoDxTM Lung (ALK) for detecting EML4-ALK fusion transcripts in the plasma of patients with non-small-cell lung cancer, ExoDx Prostate IntelliScore (EPI) as a risk assessment test for detection of high-grade prostate cancer, and MedOncAlyzer 170 a targeted pan-cancer panel for tumor profiling which examines both exosomal RNA and ctDNA. These promising tools need to be verified in larger clinical samples and populations before incorporation into routine clinical cancer diagnosis and treatment.
Recently, TEPs have found particular clinical use for early prognosis, recurrence monitoring, guiding treatment and assessing its efficacy.9,33 It has been found that there is a different mRNA expression pattern in platelets between cancer cases and healthy controls due to the tumor cells and platelets interaction. These can be used as a biomarker for early discovery of cancer or metastasis. Current clinical trials evaluate the use of platelets as liquid biopsies to guide treatment in CRC patients. They will finally clarify whether platelets as liquid biopsies have the potential to predict the prognosis of CRC cases successfully. Cancer has been shown to affect platelet count, volume, activation status, platelet-derived proteins, and RNA content, but the impact of these alterations, especially at the molecular level, has not been incorporated to routine cancer screening, diagnosis and monitoring.
Cancer and Platelets
Platelets are small (2–5 μm), specialized, hematopoietic cells without nucleus produced from megakaryocytes that circulate in the blood and are the second most prevalent cell type in blood. The mean survival time of platelets is 7-10 days after shedding from megakaryocytes. These cells, in addition to their two major functions, hemostasis and thrombosis, have crucial functions in various physiological processes involving angiogenesis, wound healing, inflammation, immune responses, neurodegenerative diseases, and cancers.34
Platelets have an important role in linking tissue damage/dysfunction and the inflammatory response during repair of injury; nevertheless, uncontrolled platelet activation results in chronic inflammation and consequently many pathological conditions, including cancer.33 Blood-based platelet parameters are potential biomarkers for many diseases, both acute and chronic, because of their easy accessibility and low-cost procedures of assessment.35 More frequently assessed platelet indices (PI) include the total platelet count (TPC), mean platelet volume (MPV) and platelet distribution width (PDW).
Strangely, for a long time, platelets have been ignored in blood biomarker studies; however, increasing evidence demonstrated that platelets have a critical role in the development and progression of diseases, especially cancer.36,37 Augmented levels of platelet count and thrombopoietic factors are often noticed in patients with cancer. However, it is unknown which one comes first, an upsurge in thrombopoietic factors induces a rise in platelet count and then tumor growth stimulation, or a tumor releases thrombopoietic factors, which results in a rise in platelet counts.38 In the presence of occult cancer, platelet generation can be severely augmented following numerous tumor-derived and systemic factors.36,39 High TPC has been documented in various cancers40; this high TPC is related to systemic inflammation,41 and increased potential risk of tumor progression in CRC.42 Thrombocytosis in adults older than 40 years could be an indicator of cancer43 and nearly 40% of cases with unknown thrombocytosis (i.e. absence of iron deficiency or inflammatory disease) harbor some form of occult cancer.44 The association between TPC and cancer is well-documented. However, the impact of TPC as a biomarker of early-stage cancer is still uncertain.
Over the lifetime of healthy people, platelet volume is quite constant but it can differ in a spectrum of diseases. It is demonstrated that platelets have elevated mitochondria and meaningfully smaller microtubules in patients with ovarian cancers in comparison to healthy people.45 MPV is shown to be increased in pancreatic cancer patients46 and raised MPV is an indicator of poor prognosis in several types of cancer including gastric cancer, pancreatic cancer and myelofibrosis.47-49 MPV, despite being an indicator of platelet size and activity, can be used as an inflammatory marker.50 Up to now, the effect of MPV in patients with cancer is not entirely elucidated. Raised MPV is associated with worse overall survival in cases with CRC51 However, reduced MPV level in cases undergoing chemotherapy is correlated with worse overall survival.52 Other studies showed that CRC patients, compared to the adenomatous polyp group, have higher MPV levels.53,54 Qian et alreported that in non-metastatic CRC, the pre- and post-treatment ratio of MPV is a prognostic factor for overall survival.55 Despite the contradictory findings of the MPV levels in cancer cases and healthy individuals, it is agreed by the majority of researches that reduced MPV is a good prognostic biomarker for cancer cases.56 Also, the impact of MPV on cancer screening seems limited to later stages of the disease.38 However, due to the slight difference between patients and controls, MPV as a sole factor does not appear to be a valuable marker.
Some studies suggested that platelet indices compared to each other or other blood variables yield better results. Studies have shown that preoperative MPV/TPC in the peripheral blood of CRC cases is low,53,57 High levels of pretreatment TPC to lymphocyte count ratio (PLR) is associated with poor overall survival in CRC patients,58 and decreased TPC/PLR shows good prognosis in patients with oligometastatic colorectal cancer.59 MPV/TPC was associated with tumor infiltration and regional lymph node metastasis in CRC.53 TPC/PLR was associated with depth of infiltration,60 and this infiltration was associated with poor prognosis in postsurgical CRC patients.61 Nowadays, some prediction models, like the ColonFlag model, have been introduced that use blood-based parameters (including PLC), age and sex combined with machine-learning techniques to differentiate high-risk from low-risk CRC patients.62,63 These models give predict CRC patients with AUC > 0.75.
Cancer and Tumor Educated Platelets
During tumor development, tumor cells with direct interaction or through several released mediators, activate (educate) platelets by changing the RNA expression profile and proteome of platelets, resulting in “TEPs”. Following signals from tumor cells, platelets modulate the splicing of their pre-mRNAs, leading to variations in their transcriptome and molecular profiles.64
Additionally, in interaction with tumor cells, platelets during their life cycle pick up biomolecules released from these cells and constantly absorb and enrich circulating free proteins, vesicles, nucleic acids and particles.33 The surface of platelets is crucial for communication between platelets and other cells in the plasma due to their active biomolecules on the surface. This indicates that platelets can be “educated” by numerous cell types, for example erythrocytes, leukocytes, megakaryocytes, and tumor cells.65
The role of TEPs in the progression and metastasis of numerous solid tumors is documented. Studies on nearly all different types of cancer have shown that the tumor induces activation of the clotting cascade through interactions with platelets.9 Cancer cells, through a key process called tumor cell-induced platelet aggregation, induce platelets to be activated, aggregated, and produce tumor favorite particles (Figure 1).41
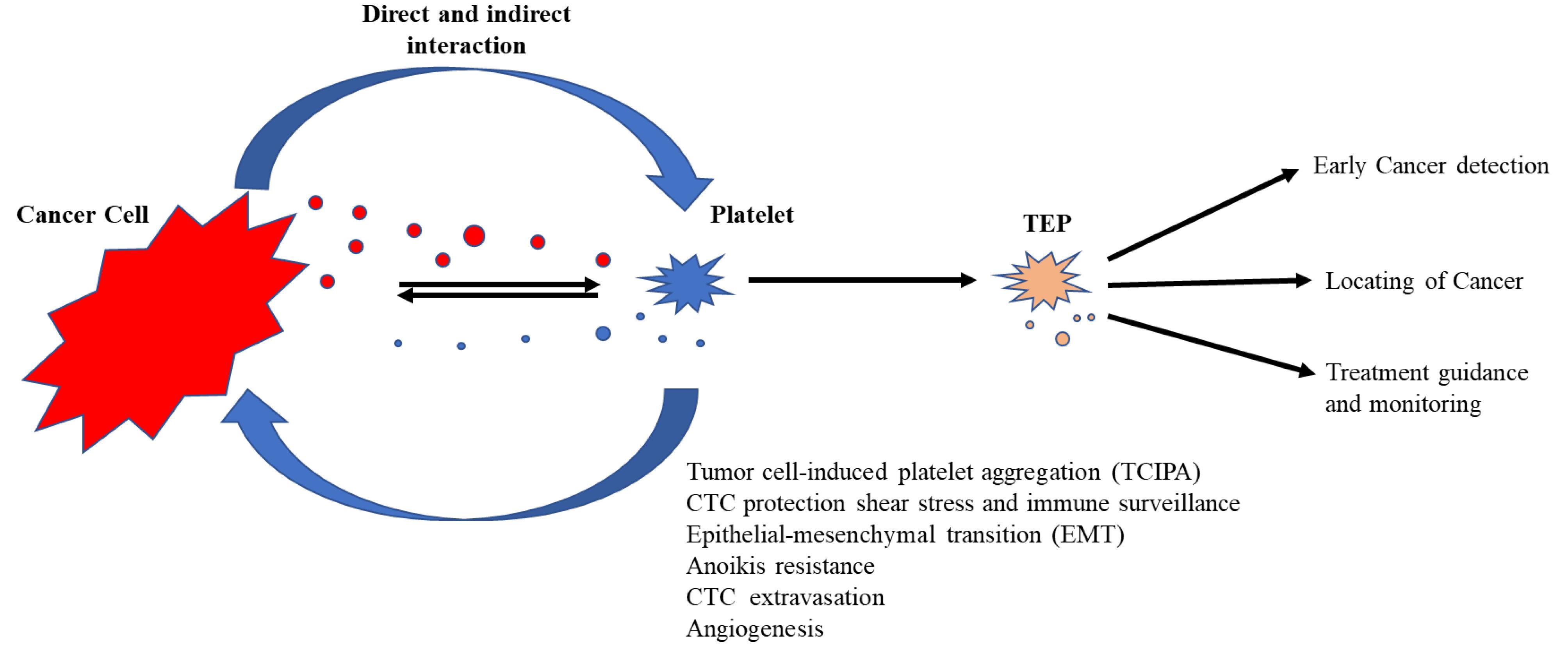
Figure 1.
Activated Tumor-Educated Platelets (TEPs) Produced by Reciprocal Tumor-Platelet Interaction
.
Activated Tumor-Educated Platelets (TEPs) Produced by Reciprocal Tumor-Platelet Interaction
Cancer cells survive via platelet-mediated protection after entering the circulation system and subsequently, CTCs engage in extravasation, angiogenesis/vascular permeability and anoikis resistance.34 The communication between platelets and tumor is obviously mutual.66 Platelets were shown to have a stimulatory outcome on tumor progression,36,37 whereas tumor modulates multiple platelet features and functions and these alterations seem to be in the early stages of malignancy.67-69
Platelets may communicate with other cells through discharging two types of EVs, exosomes (40–100 nm) and microparticles (100–1000 nm). Platelet-derived microparticles are the most prevalent EVs that exist in the blood.70 In physiological conditions, platelet-derived EVs are mostly generated from megakaryocytes71; however, induced platelet-derived EVs are associated with platelet activation.72 Medium-sized EVs (mEVs) secreted by thrombin-induced platelets of CRC cases showed different size distribution, and proteomic profiles compared to healthy subjects and stimulated prometastatic and prothrombotic phenotypes in tumor cells.73
TEPs in Colorectal Cancer
The interaction of platelets and cancers is complex and the mechanism has not been fully discovered. Plantureux et al demonstrated that the CRC tumor cells’ interaction with platelets generates chimeric EVs that inhibit early tumor growth through triggering macrophages, while promoting metastasis by epithelial-mesenchymal transition and endothelial activation.74 Recently, TEPs have been found to hold potential value as a promising liquid biopsy for several clinical and research uses.75 Platelet transcriptomes have numerous superiorities as a source for liquid biopsies in cancer management. Storing whole blood for up to 48 hours at room temperature does not have a significant impact on platelet RNA profiles. Abundant platelets are easily isolated and contain high-quality RNA. Distinct from nucleated cells with an active transcriptional machine, platelets transcriptomes are generally unchanged during the isolation process.75 Platelets are extremely reactive cells and may provide high sensitivity for longitudinal studies in cancer. TEPs can provide information on the existence, site, and molecular features of tumors by TEPs RNA splicing signatures.76 Although it is not completely discovered what factors exactly lead to differential transcriptional characteristics in platelets from patients with tumor, they are likely due to pick up of tumor-derived and circulating RNAs and reflect influences of the tumor microenvironment.77,78
Most of the studies on the platelets of CRC patients have focused on mRNA expression analysis. These studies used RNA-seq to profile mRNAs of TEPs in CRC patients,68,79,80 and four studies used their data re-analysis and validation in CRC tissue,81 serum82 and platelets83,84 (Table 2). Best et al in 2015, in search for pan-cancer biomarkers, studied the potential of TEP mRNA profiles in several cancer patients and healthy donors from different cancer types. Using RNA-seq analysis, they could differentiate cancer patients from healthy volunteers. Additionally, this process precisely distinguished driver mutations, consisting of KRAS-, PIK3C4-, EGFR-, HER-2 and MET-positive tumors, proposing that TEPs may provide an accurate means for cancer detection and targeted treatments.68 Zou et al in 2022 constructed a platelet-based gene expression database, PltDB,85 that includes mRNAs, lncRNAs, miRNAs, and circRNA from 29 disease type (including CRC), 1,736 patients, and 672 controls from their previous RNA-seq data.79 They demonstrated that RNA profiles could be used for distinguishing and also cancer staging of CRC cases from other intestinal diseases unrelated to cancer.79
Table 2.
Reported Studies in Tumor-Educated Platelets of CRC Patients
|
Ref/Year
|
Samples
|
Method
|
Biomarker
|
Results
|
| 2012 70 |
Platelet & Plasma
35 CRC, 84 HC |
ELISA |
VEGF, bFGF, PDGF, PF4, TSP-1 |
VEGF, PDGF and PF4 high in platelet, not plasma |
| 2015 71 |
288 platelet samples from 6 cancer types including
41 CRC, 55 HC |
RNA-seq |
mRNA |
Distinguishing cancer with 96% accuracy, identifying site of primary tumor with 71% accuracy
GEO: GSE68086 |
| 2019 85 |
Platelet
286 CRC (I66, II162, III23, IV27), 41 HC |
Bioinformatic analysis (GSE68086)
& qRT-PCR, functionally validation |
mRNA |
TIMP1 a potential independent diagnostic biomarker,
platelets could carry RNA to CRC cells |
| 2021 86 |
Platelet
19 CRC, 4 HC |
Bioinformatic analysis (GSE68086 and GSE89843),
qRT-PCR |
mRNA |
7-gene prognostic model including RSL24D1 ↓, IFI27, HBD, CRYM, FCGR2A, IFITM3, and KLHDC8B ↑ |
| 2022 82 |
1628 platelet samples from 18 cancer types including
85 CRC, 390 HC |
RNA-seq |
mRNA |
Average 75% specificity, accurate detection in
(stage I-IV), ½ (stage I-III), tumor site detection in 80% of patients
GEO: GSE183635 |
| 2022 83 |
Tissue
Discovery (245 CRC), Validation (191 CRC) |
Bioinformatic analysis (GSE161158, TCGA) of CRC-specific platelet-related genes |
mRNA |
10-gene prognostic model including TIAL1, C1orf198, PTPRN, CLK1, ANKRD13D, LSMEM1, ATP6AP1, SKAP1 ↑, ERAP1 and ANKRD17 ↓ |
| 2022 81 |
Platelet, 132 CRC (I25, II48, III58, IV1),
190 HC |
RNA-seq |
mRNA |
AUC = 0.92
BioProject Accession: PRJNA737596 |
| 2022 84 |
Serum
105 CRC, 105 HC |
Bioinformatic analysis (GSE68086) & qRT-PCR of
Platelet-associated lncRNAs |
LncRNA |
LNCAROD, LINC00534, SNHG20, and TSPOAP-AS1 ↑ (both platelet and serum), AUC = 0.78 |
Interestingly, one study investigated differentially expressed lncRNAs of CRC-platelets in the serum of CRC patients.82 They used RNA-seq data (GEO: GSE68086),68 to investigate differentially expressed lncRNAs in CRC-platelet samples. They then selected the four most overexpressed and the four most suppressed lncRNAs for final investigations. With a training set of 45 CRC (26 stage I/II, 18 stage III/IV) and 45 HC, and a validation set of 105 CRC (52 stage I/II, 52 stage III/IV) and 105 HC, they demonstrated that four lncRNAs, SNHG20, LNCAROD, LINC00534, and TSPOAP-AS1, were overexpressed in both the platelets and serum of CRC patients and these four lncRNAs can predict CRC with an AUC of 0.78; the expression levels of TSPOAP-AS1 and LNCAROD were associated with the staging of cancer and location of tumor.
Platelets after activation can transfer many bioactive proteins into the microenvironment and uptake tumor-derived factors from the microenvironment; so the platelet proteome is significantly changed in cancer patients.56 Accordingly, PDGF, PF-4 and VEGF elevations were demonstrated in the platelets of CRC patients. These platelet-related proteins can be independent factors for predicting and significantly distinguishing CRC (AUC: 0.893, P < 0.001).
In conclusion, in the therapy of CRC, early diagnosis, particularly the identification of precancerous adenomas, is believed to be crucial for improving patient survival. It is shown that common clinical features of platelets including TPC and MPV are changed in patients with cancer; however, their potential as diagnostic biomarkers for early cancer detection need extra investigations for confirmation. Liquid biopsy is a promising tool for cancer screening and early cancer detection and is an effective alternative or complement to the biopsy of tissue that enables the improvement of the overall survival of cancer patients. Generally, liquid biopsy is noninvasive, resolves heterogeneity of tumor and can provide real-time monitoring of cancer progression, treatment response or recurrence. Investigation of platelet (TEPs) has demonstrated that these cells could be a valuable tool for liquid biopsy of cancer patients, similar to other fluid biopsy tools (e.g. CTC, exosomes, ctDNA, cfRNA).
The application of TEPs RNA profile in combination with artificial intelligence has shown high promise in the finding of CRC tumor and staging. However, the participation of TEP in CRC progression is not completely apparent, and their role as a tumoral marker requires further studies.
Further protein and RNA profiles of TEPs from various cancers with different stages and long-term follow-up are needed for them to be used accurately with high sensitivity and specificity in the detection of early cancer and determining tumor stages. Also, combinatory analysis of TEP RNA with complementary sources, such as cfDNA/RNA and exosomes, will improve the detection of CRC in an early stage and help noninvasive disease observation. Imaging of platelet subcellular structures with super-resolution fluorescence microscopy is another advanced technology in the field of liquid biopsy that may open new horizons for clinical applications.86
Competing Interests
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
References
- Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther 2020; 5(1):22. doi: 10.1038/s41392-020-0116-z [Crossref] [ Google Scholar]
- Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021; 134(7):783-91. doi: 10.1097/cm9.0000000000001474 [Crossref] [ Google Scholar]
- Yang Y, Zhu H, Li Q. Partial response of donafenib as the third-line therapy in metastatic colon cancer: a case report. Medicine (Baltimore) 2021; 100(37):e27204. doi: 10.1097/md.0000000000027204 [Crossref] [ Google Scholar]
- Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK. Colon cancer, version 22021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021; 19(3):329-59. doi: 10.6004/jnccn.2021.0012 [Crossref] [ Google Scholar]
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66(4):683-91. doi: 10.1136/gutjnl-2015-310912 [Crossref] [ Google Scholar]
- Shaukat A, Levin TR. Current and future colorectal cancer screening strategies. Nat Rev Gastroenterol Hepatol 2022; 19(8):521-31. doi: 10.1038/s41575-022-00612-y [Crossref] [ Google Scholar]
- Liu L, Lin F, Ma X, Chen Z, Yu J. Tumor-educated platelet as liquid biopsy in lung cancer patients. Crit Rev Oncol Hematol 2020; 146:102863. doi: 10.1016/j.critrevonc.2020.102863 [Crossref] [ Google Scholar]
- Liu Z, Kong Y, Dang Q, Weng S, Zheng Y, Ren Y. Liquid biopsy in pre-metastatic niche: from molecular mechanism to clinical application. Front Immunol 2022; 13:958360. doi: 10.3389/fimmu.2022.958360 [Crossref] [ Google Scholar]
- Zhou H, Zhu L, Song J, Wang G, Li P, Li W. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol Cancer 2022; 21(1):86. doi: 10.1186/s12943-022-01556-2 [Crossref] [ Google Scholar]
- Wu J, Hu S, Zhang L, Xin J, Sun C, Wang L. Tumor circulome in the liquid biopsies for cancer diagnosis and prognosis. Theranostics 2020; 10(10):4544-56. doi: 10.7150/thno.40532 [Crossref] [ Google Scholar]
- Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med 2018; 379(18):1754-65. doi: 10.1056/NEJMra1706174 [Crossref] [ Google Scholar]
- Antunes-Ferreira M, Koppers-Lalic D, Würdinger T. Circulating platelets as liquid biopsy sources for cancer detection. Mol Oncol 2021; 15(6):1727-43. doi: 10.1002/1878-0261.12859 [Crossref] [ Google Scholar]
- Mazouji O, Ouhajjou A, Incitti R, Mansour H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front Cell Dev Biol 2021; 9:660924. doi: 10.3389/fcell.2021.660924 [Crossref] [ Google Scholar]
- Joosse SA, Pantel K. Tumor-educated platelets as liquid biopsy in cancer patients. Cancer Cell 2015; 28(5):552-4. doi: 10.1016/j.ccell.2015.10.007 [Crossref] [ Google Scholar]
- Raza A, Khan AQ, Inchakalody VP, Mestiri S, Yoosuf Z, Bedhiafi T. Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer. J Exp Clin Cancer Res 2022; 41(1):99. doi: 10.1186/s13046-022-02318-0 [Crossref] [ Google Scholar]
- Burz C, Rosca A, Pop VV, Buiga R, Aldea C, Samasca G. Liquid biopsy challenge and hope in colorectal cancer. Expert Rev Mol Diagn 2019; 19(4):341-8. doi: 10.1080/14737159.2019.1597708 [Crossref] [ Google Scholar]
- Mamdani H, Ahmed S, Armstrong S, Mok T, Jalal SI. Blood-based tumor biomarkers in lung cancer for detection and treatment. Transl Lung Cancer Res 2017; 6(6):648-60. doi: 10.21037/tlcr.2017.09.03 [Crossref] [ Google Scholar]
- Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 2004; 10(24):8152-62. doi: 10.1158/1078-0432.ccr-04-1110 [Crossref] [ Google Scholar]
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol 2009; 20(7):1223-9. doi: 10.1093/annonc/mdn786 [Crossref] [ Google Scholar]
- CELLSEARCH®. Available from: https://www.cellsearchctc.com/. Accessed September 2023.
- Cayrefourcq L, Mazard T, Joosse S, Solassol J, Ramos J, Assenat E. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res 2015; 75(5):892-901. doi: 10.1158/0008-5472.can-14-2613 [Crossref] [ Google Scholar]
- García-Pardo M, Makarem M, Li JJN, Kelly D, Leighl NB. Integrating circulating-free DNA (cfDNA) analysis into clinical practice: opportunities and challenges. Br J Cancer 2022; 127(4):592-602. doi: 10.1038/s41416-022-01776-9 [Crossref] [ Google Scholar]
- Birkó Z, Nagy B, Klekner Á, Virga J. Novel molecular markers in glioblastoma-benefits of liquid biopsy. Int J Mol Sci 2020; 21(20):7522. doi: 10.3390/ijms21207522 [Crossref] [ Google Scholar]
- Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001; 61(4):1659-65. [ Google Scholar]
- Spindler KL, Appelt AL, Pallisgaard N, Andersen RF, Brandslund I, Jakobsen A. Cell-free DNA in healthy individuals, noncancerous disease and strong prognostic value in colorectal cancer. Int J Cancer 2014; 135(12):2984-91. doi: 10.1002/ijc.28946 [Crossref] [ Google Scholar]
- Mouliere F, El Messaoudi S, Pang D, Dritschilo A, Thierry AR. Multi-marker analysis of circulating cell-free DNA toward personalized medicine for colorectal cancer. Mol Oncol 2014; 8(5):927-41. doi: 10.1016/j.molonc.2014.02.005 [Crossref] [ Google Scholar]
- Styk J, Buglyó G, Pös O, Csók Á, Soltész B, Lukasz P. Extracellular nucleic acids in the diagnosis and progression of colorectal cancer. Cancers (Basel) 2022; 14(15):3712. doi: 10.3390/cancers14153712 [Crossref] [ Google Scholar]
- Schiffman JD, Fisher PG, Gibbs P. Early detection of cancer: past, present, and future. Am Soc Clin Oncol Educ Book 2015; 35(1):57-65. doi: 10.14694/EdBook_AM.2015.35.57 [Crossref] [ Google Scholar]
- Jiang L, Gu Y, Du Y, Liu J. Exosomes: diagnostic biomarkers and therapeutic delivery vehicles for cancer. Mol Pharm 2019; 16(8):3333-49. doi: 10.1021/acs.molpharmaceut.9b00409 [Crossref] [ Google Scholar]
- Liu J, Ren L, Li S, Li W, Zheng X, Yang Y. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B 2021; 11(9):2783-97. doi: 10.1016/j.apsb.2021.01.001 [Crossref] [ Google Scholar]
- Yu D, Li Y, Wang M, Gu J, Xu W, Cai H. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer 2022; 21(1):56. doi: 10.1186/s12943-022-01509-9 [Crossref] [ Google Scholar]
- Halvaei S, Daryani S, Eslami Samarin S, Samadi T, Jafarbeik-Iravani N, Oghabi Bakhshayesh T. Exosomes in cancer liquid biopsy: a focus on breast cancer. Mol Ther Nucleic Acids 2018; 10:131-41. doi: 10.1016/j.omtn.2017.11.014 [Crossref] [ Google Scholar]
- Dovizio M, Ballerini P, Fullone R, Tacconelli S, Contursi A, Patrignani P. Multifaceted functions of platelets in cancer: from tumorigenesis to liquid biopsy tool and drug delivery system. Int J Mol Sci 2020; 21(24):9585. doi: 10.3390/ijms21249585 [Crossref] [ Google Scholar]
- Wang L, Wang X, Guo E, Mao X, Miao S. Emerging roles of platelets in cancer biology and their potential as therapeutic targets. Front Oncol 2022; 12:939089. doi: 10.3389/fonc.2022.939089 [Crossref] [ Google Scholar]
- Lembeck AL, Posch F, Klocker EV, Szkandera J, Schlick K, Stojakovic T. Large platelet size is associated with poor outcome in patients with metastatic pancreatic cancer. Clin Chem Lab Med 2019; 57(5):740-4. doi: 10.1515/cclm-2018-0016 [Crossref] [ Google Scholar]
- Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell 2018; 33(6):965-83. doi: 10.1016/j.ccell.2018.03.002 [Crossref] [ Google Scholar]
- Plantureux L, Crescence L, Dignat-George F, Panicot-Dubois L, Dubois C. Effects of platelets on cancer progression. Thromb Res 2018; 164 Suppl 1:S40-S7. doi: 10.1016/j.thromres.2018.01.035 [Crossref] [ Google Scholar]
- Sabrkhany S, Kuijpers MJE, Oude Egbrink MGA, Griffioen AW. Platelets as messengers of early-stage cancer. Cancer Metastasis Rev 2021; 40(2):563-73. doi: 10.1007/s10555-021-09956-4 [Crossref] [ Google Scholar]
- Lin RJ, Afshar-Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood 2014; 124(2):184-7. doi: 10.1182/blood-2014-03-562538 [Crossref] [ Google Scholar]
- Meng Y, Sun J, Zheng Y, Zhang G, Yu T, Piao H. Platelets: the emerging clinical diagnostics and therapy selection of cancer liquid biopsies. Onco Targets Ther 2021; 14:3417-28. doi: 10.2147/ott.s311907 [Crossref] [ Google Scholar]
- Väyrynen JP, Väyrynen SA, Sirniö P, Minkkinen I, Klintrup K, Karhu T. Platelet count, aspirin use, and characteristics of host inflammatory responses in colorectal cancer. J Transl Med 2019; 17(1):199. doi: 10.1186/s12967-019-1950-z [Crossref] [ Google Scholar]
- Sylman JL, Boyce HB, Mitrugno A, Tormoen GW, Thomas IC, Wagner TH. A temporal examination of platelet counts as a predictor of prognosis in lung, prostate, and colon cancer patients. Sci Rep 2018; 8(1):6564. doi: 10.1038/s41598-018-25019-1 [Crossref] [ Google Scholar]
- Bailey SE, Ukoumunne OC, Shephard EA, Hamilton W. Clinical relevance of thrombocytosis in primary care: a prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br J Gen Pract 2017; 67(659):e405-e13. doi: 10.3399/bjgp17X691109 [Crossref] [ Google Scholar]
- Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med 1964; 114:497-500. doi: 10.1001/archinte.1964.03860100079008 [Crossref] [ Google Scholar]
- Wang R, Stone RL, Kaelber JT, Rochat RH, Nick AM, Vijayan KV. Electron cryotomography reveals ultrastructure alterations in platelets from patients with ovarian cancer. Proc Natl Acad Sci U S A 2015; 112(46):14266-71. doi: 10.1073/pnas.1518628112 [Crossref] [ Google Scholar]
- Sabrkhany S, Kuijpers MJE, van Kuijk SMJ, Sanders L, Pineda S, Olde Damink SWM. A combination of platelet features allows detection of early-stage cancer. Eur J Cancer 2017; 80:5-13. doi: 10.1016/j.ejca.2017.04.010 [Crossref] [ Google Scholar]
- Kılınçalp S, Ekiz F, Başar O, Ayte MR, Coban S, Yılmaz B. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets 2014; 25(8):592-4. doi: 10.3109/09537104.2013.783689 [Crossref] [ Google Scholar]
- Yin JB, Wang X, Zhang X, Liu L, Wang RT. Mean platelet volume predicts survival in pancreatic cancer patients with synchronous liver metastases. Sci Rep 2018; 8(1):6014. doi: 10.1038/s41598-018-24539-0 [Crossref] [ Google Scholar]
- Lucijanic M, Mitrovic Z, Cicic D, Prka Z, Pejsa V, Livun A. Increased mean platelet volume (MPV) is an independent predictor of inferior survival in patients with primary and secondary myelofibrosis. Int J Hematol 2018; 107(2):166-72. doi: 10.1007/s12185-017-2348-4 [Crossref] [ Google Scholar]
- Korniluk A, Koper-Lenkiewicz OM, Kamińska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm 2019; 2019:9213074. doi: 10.1155/2019/9213074 [Crossref] [ Google Scholar]
- Li N, Yu Z, Zhang X, Liu T, Sun YX, Wang RT. Elevated mean platelet volume predicts poor prognosis in colorectal cancer. Sci Rep 2017; 7(1):10261. doi: 10.1038/s41598-017-11053-y [Crossref] [ Google Scholar]
- Chang J, Lin G, Ye M, Tong D, Zhao J, Zhu D. Decreased mean platelet volume predicts poor prognosis in metastatic colorectal cancer patients treated with first-line chemotherapy: results from mCRC biomarker study. BMC Cancer 2019; 19(1):15. doi: 10.1186/s12885-018-5252-2 [Crossref] [ Google Scholar]
- Zhang H, Lin F, Wang Z. Mean platelet volume/platelet count ratio in combination with tumor markers in colorectal cancer: a retrospective clinical study. BMC Cancer 2023; 23(1):124. doi: 10.1186/s12885-023-10585-z [Crossref] [ Google Scholar]
- Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman Vujacic I. Combined diagnostic efficacy of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV) as biomarkers of systemic inflammation in the diagnosis of colorectal cancer. Dis Markers 2019; 2019:6036979. doi: 10.1155/2019/6036979 [Crossref] [ Google Scholar]
- Qian W, Ge XX, Wu J, Gong FR, Wu MY, Xu MD. Prognostic evaluation of resectable colorectal cancer using platelet-associated indicators. Oncol Lett 2019; 18(1):571-80. doi: 10.3892/ol.2019.10388 [Crossref] [ Google Scholar]
- Chen M, Hou L, Hu L, Tan C, Wang X, Bao P. Platelet detection as a new liquid biopsy tool for human cancers. Front Oncol 2022; 12:983724. doi: 10.3389/fonc.2022.983724 [Crossref] [ Google Scholar]
- Wu YY, Zhang X, Qin YY, Qin JQ, Lin FQ. Mean platelet volume/platelet count ratio in colorectal cancer: a retrospective clinical study. BMC Cancer 2019; 19(1):314. doi: 10.1186/s12885-019-5504-9 [Crossref] [ Google Scholar]
- Wang J, Li J, Wei S, Xu J, Jiang X, Yang L. The ratio of platelets to lymphocytes predicts the prognosis of metastatic colorectal cancer: a review and meta-analysis. Gastroenterol Res Pract 2021; 2021:9699499. doi: 10.1155/2021/9699499 [Crossref] [ Google Scholar]
- Huang X, Cui J, Li X, Liu C, Sun J, Yue J. The decreased platelet-to-lymphocyte ratio could predict a good prognosis in patients with oligometastatic colorectal cancer: a single-center cohort retrospective study. World J Surg Oncol 2021; 19(1):297. doi: 10.1186/s12957-021-02406-z [Crossref] [ Google Scholar]
- Tan D, Fu Y, Su Q, Wang H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2016; 95(24):e3837. doi: 10.1097/md.0000000000003837 [Crossref] [ Google Scholar]
- Miao Y, Xu Z, Feng W, Zheng M, Xu Z, Gao H. Platelet infiltration predicts survival in postsurgical colorectal cancer patients. Int J Cancer 2022; 150(3):509-20. doi: 10.1002/ijc.33816 [Crossref] [ Google Scholar]
- Goshen R, Choman E, Ran A, Muller E, Kariv R, Chodick G. Computer-assisted flagging of individuals at high risk of colorectal cancer in a large health maintenance organization using the ColonFlag test. JCO Clin Cancer Inform 2018; 2:1-8. doi: 10.1200/cci.17.00130 [Crossref] [ Google Scholar]
- Virdee PS, Patnick J, Watkinson P, Holt T, Birks J. Full blood count trends for colorectal cancer detection in primary care: development and validation of a dynamic prediction model. Cancers (Basel) 2022; 14(19):4779. doi: 10.3390/cancers14194779 [Crossref] [ Google Scholar]
- Wang B, Li F, Cheng L, Zhan Y, Wu B, Chen P. The pretreatment platelet count is an independent predictor of tumor progression in patients undergoing transcatheter arterial chemoembolization with hepatitis B virus-related hepatocellular carcinoma. Future Oncol 2019; 15(8):827-39. doi: 10.2217/fon-2018-0591 [Crossref] [ Google Scholar]
- Patel D, Thankachan S, Sreeram S, Kavitha KP, Suresh PS. The role of tumor-educated platelets in ovarian cancer: a comprehensive review and update. Pathol Res Pract 2023; 241:154267. doi: 10.1016/j.prp.2022.154267 [Crossref] [ Google Scholar]
- Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelet-cancer interactions. Semin Thromb Hemost 2014; 40(3):296-305. doi: 10.1055/s-0034-1370767 [Crossref] [ Google Scholar]
- Peterson JE, Zurakowski D, Italiano JE Jr, Michel LV, Connors S, Oenick M. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis 2012; 15(2):265-73. doi: 10.1007/s10456-012-9259-z [Crossref] [ Google Scholar]
- Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F. RNA-Seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell 2015; 28(5):666-76. doi: 10.1016/j.ccell.2015.09.018 [Crossref] [ Google Scholar]
- Sabrkhany S, Kuijpers MJE, Knol JC, Olde Damink SWM, Dingemans AC, Verheul HM. Exploration of the platelet proteome in patients with early-stage cancer. J Proteomics 2018; 177:65-74. doi: 10.1016/j.jprot.2018.02.011 [Crossref] [ Google Scholar]
- Flaumenhaft R, Mairuhu AT, Italiano JE. Platelet- and megakaryocyte-derived microparticles. Semin Thromb Hemost 2010; 36(8):881-7. doi: 10.1055/s-0030-1267042 [Crossref] [ Google Scholar]
- Flaumenhaft R, Dilks JR, Richardson J, Alden E, Patel-Hett SR, Battinelli E. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood 2009; 113(5):1112-21. doi: 10.1182/blood-2008-06-163832 [Crossref] [ Google Scholar]
- Taus F, Meneguzzi A, Castelli M, Minuz P. Platelet-derived extracellular vesicles as target of antiplatelet agents. What is the evidence? Front Pharmacol 2019; 10:1256. doi: 10.3389/fphar.2019.01256 [Crossref] [ Google Scholar]
- Contursi A, Fullone R, Szklanna-Koszalinska P, Marcone S, Lanuti P, Taus F. Tumor-educated platelet extracellular vesicles: proteomic profiling and crosstalk with colorectal cancer cells. Cancers (Basel) 2023; 15(2):350. doi: 10.3390/cancers15020350 [Crossref] [ Google Scholar]
- Plantureux L, Mège D, Crescence L, Carminita E, Robert S, Cointe S. The interaction of platelets with colorectal cancer cells inhibits tumor growth but promotes metastasis. Cancer Res 2020; 80(2):291-303. doi: 10.1158/0008-5472.can-19-1181 [Crossref] [ Google Scholar]
- Krishnan A, Thomas S. Toward platelet transcriptomics in cancer diagnosis, prognosis and therapy. Br J Cancer 2022; 126(3):316-22. doi: 10.1038/s41416-021-01627-z [Crossref] [ Google Scholar]
- Best MG, Wesseling P, Wurdinger T. Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res 2018; 78(13):3407-12. doi: 10.1158/0008-5472.can-18-0887 [Crossref] [ Google Scholar]
- Roweth HG, Battinelli EM. Lessons to learn from tumor-educated platelets. Blood 2021; 137(23):3174-80. doi: 10.1182/blood.2019003976 [Crossref] [ Google Scholar]
- Davizon-Castillo P, Rowley JW, Rondina MT. Megakaryocyte and platelet transcriptomics for discoveries in human health and disease. Arterioscler Thromb Vasc Biol 2020; 40(6):1432-40. doi: 10.1161/atvbaha.119.313280 [Crossref] [ Google Scholar]
- Xu L, Li X, Li X, Wang X, Ma Q, She D. RNA profiling of blood platelets noninvasively differentiates colorectal cancer from healthy donors and noncancerous intestinal diseases: a retrospective cohort study. Genome Med 2022; 14(1):26. doi: 10.1186/s13073-022-01033-x [Crossref] [ Google Scholar]
- In ‘t Veld S, Arkani M, Post E, Antunes-Ferreira M, D’Ambrosi S, Vessies DCL, et al. Detection and localization of early- and late-stage cancers using platelet RNA. Cancer Cell 2022;40(9):999-1009.e6. 10.1016/j.ccell.2022.08.006.
- Wang P, Zhao W, Cao H. Development of a platelet-related prognostic model for colorectal cancer. Front Genet 2022; 13:904168. doi: 10.3389/fgene.2022.904168 [Crossref] [ Google Scholar]
- Ye B, Li F, Chen M, Weng Y, Qi C, Xie Y. A panel of platelet-associated circulating long non-coding RNAs as potential biomarkers for colorectal cancer. Genomics 2022; 114(1):31-7. doi: 10.1016/j.ygeno.2021.11.026 [Crossref] [ Google Scholar]
- Yang L, Jiang Q, Li DZ, Zhou X, Yu DS, Zhong J. TIMP1 mRNA in tumor-educated platelets is diagnostic biomarker for colorectal cancer. Aging (Albany NY) 2019; 11(20):8998-9012. doi: 10.18632/aging.102366 [Crossref] [ Google Scholar]
- Ge X, Yuan L, Cheng B, Dai K. Identification of seven tumor-educated platelets RNAs for cancer diagnosis. J Clin Lab Anal 2021; 35(6):e23791. doi: 10.1002/jcla.23791 [Crossref] [ Google Scholar]
- Zou D, Yuan Y, Xu L, Lei S, Li X, Lu X. PltDB: a blood platelets-based gene expression database for disease investigation. Bioinformatics 2022; 38(11):3143-5. doi: 10.1093/bioinformatics/btac278 [Crossref] [ Google Scholar]
- Xu P, Deng H, Hong Z, Zhong S, Chen F, Wang L, et al. Superresolution fluorescence microscopy of platelet subcellular structures as a potential tumor liquid biopsy. Small Methods. 2023:e2300445. 10.1002/smtd.202300445.