Arch Iran Med. 26(5):252-260.
doi: 10.34172/aim.2023.39
Original Article
Cardio-Pulmonary Histopathology with Clinical Correlations of Deceased Patients with COVID-19: A Case Series in Tehran, Iran
Saeed Soleiman-Meigooni Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing, 1, * 
Ramin Yaghmayee Conceptualization, Validation, 2
Shadi Mohammadi Data curation, Investigation, 3
Mousa Ahmadi Data curation, 4
Mehdi Sakhabakhsh Data curation, 5
Ramin Hamidi-Farahani Funding acquisition, Resources, 4
Ebrahim Hazrati Funding acquisition, Resources, 6
Seyed Mohammad Jazayeri Writing – original draft, 7
Mahtab Fotoohi Data curation, Validation, 2
Akram Motemaveleh Data curation, 8
Vahid Doulatabadi-Farahani Data curation, 9
Farhad Shahmohamadi Data curation, Supervision, 10
Mohammad Hassan Kazemi-Galougahi Formal analysis, Methodology, 11
Ali Asgari Data curation, 4
Mohammad Aminianfar Data curation, 4
Mohammad Darvishi Data curation, 4
Mojgan Mohajeri-Iravani Data curation, 12
Omid Gholizadeh Software, Visualization, 13
Author information:
1Infectious Diseases Research Center, Aja University of Medical Sciences, Tehran, Iran
2Department of Pathology, Khanevadeh University Hospital, Aja University of Medical Sciences, Tehran, Iran
3Department of Obstetrics and Gynecology, Khanevadeh University Hospital, Aja University of Medical Sciences, Tehran, Iran
4Department of Infectious Diseases, Faculty of Medicine, Aja University of Medical Sciences, Tehran, Iran
5Department of Neurology, Faculty of Medicine, Aja University of Medical Sciences, Tehran, Iran
6Department of Anesthesiology, Faculty of Medicine, Aja University of Medical Sciences, Tehran, Iran
7Research Center for Clinical Virology, Tehran University of Medical Sciences, Tehran, Iran
8Department of Pulmonology, Khanevadeh University Hospital, Aja University of Medical Sciences, Tehran, Iran
9Department of Cardiology, Khanevadeh University Hospital, Aja University of Medical Sciences, Tehran, Iran
10Department of Forensic Medicine, Khanevadeh University Hospital, Aja University of Medical Sciences, Tehran, Iran
11Department of Epidemiology, Faculty of Medicine, Aja University of Medical Sciences, Tehran, Iran
12Department of Anesthesiology, Faculty of Paramedical Sciences, Aja University of Medical Sciences, Tehran, Iran
13Research Center for Clinical Virology, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Background:
SARS-CoV-2 may affect vital organs. The present study investigated the histopathology of pulmonary and cardiac tissues with clinical correlation in deceased patients with COVID-19.
Methods:
We obtained pulmonary and cardiac tissues from 30 deceased patients with COVID-19 in Tehran, Iran, from January to May 2021. Sampling was performed through a percutaneous needle biopsy. After slide preparation, two expert pathologists studied them. We assessed the correlation between clinical and pathological data by Fisher’s exact test.
Results:
The mean age of the patients was 73.8±13.4 years, and the male-to-female ratio was 23/7. The most common underlying disease was hypertension (HTN) in 25 patients (83%). Fifty-five tissue samples were achieved, including 28 pulmonary and 27 cardiac samples. Our results showed that all patients (100%) developed diffuse alveolar damage (DAD), and 26 (93%) developed hyaline membrane formation. The most common phase of DAD was the exudative-proliferative phase in 16 (57.1%). Three cardiac samples (11%) revealed myocarditis, and seven (26%) showed cardiomyocyte hypertrophy. In univariate analysis using Fischer’s exact test, myocarditis had significant relationships with C-reactive protein (CRP) levels higher than 80 mg/dL (P=0.008) and elevated cardiac troponin levels higher than two-fold (P=0.01).
Conclusion:
COVID-19 can affect the major vital organs. However, only myocarditis had a significant relationship with the circulating levels of inflammatory factors.
Keywords: Cardiac, Pathology, Pulmonary, SARS-CoV-2
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Soleiman-Meigooni S, Yaghmayee R, Mohammadi S, Ahmadi M, Sakhabakhsh M, Hamidi-Farahani R, et al. Cardio-pulmonary histopathology with clinical correlations of deceased patients with COVID-19: a case series in Tehran, Iran. Arch Iran Med. 2023;26(5):252-260. doi: 10.34172/aim.2023.39
Introduction
SARS-CoV-2 enters the respiratory epithelial cells via angiotensin-converting enzyme II (ACE-II) receptors.1 Several chronic diseases such as diabetes mellitus (DM), hypertension (HTN), chronic lung disease (CLD), chronic renal failure (CRF), and ischemic heart disease (IHD) lead to increased expression of ACE-II receptors, higher viral binding, and a more severe immune response. This phenomenon explains the severe COVID-19 in the elderly with underlying diseases.2,3 The primary organ target of this virus is the lungs. In severe disease, respiratory distress and diffuse alveolar damage (DAD) constitute the leading cause of death.4 COVID-19 can affect other vital organs and cause fatal inflammation. Numerous cases of myocarditis and encephalitis have been reported in patients with COVID-19.5-7 Nevertheless, only a few studies performed multi-organ biopsies on these patients. We aimed to assess the clinical and histopathological features of pulmonary and cardiac tissues in deceased patients with COVID-19.
Materials and Methods
Study Design
We performed this case series on deceased patients with a polymerase chain reaction-confirmed COVID-19 in Khanevadeh Academic Hospital in Tehran, Iran, from January to May 2021.We selected our subjects by conventional sampling.
Participants and Sampling Methods
We only selected deceased patients whose first-degree relatives gave written informed consent for sampling in that period. The bodies underwent minimal invasive percutaneous needle biopsy 6‒24 hours following death. An expert with a universal gun, two coats of gloves, a five-layered mask, and a face shield took the samples in a dissection room with negative pressure ventilation.8 A semi-automatic biopsy needle was used for cardiac and pulmonary sampling (Supplementary file 1, Figure S1). The pulmonary samples were taken from the areas with the most severe involvement on the computed tomography scan, often through the seventh or eighth intercostal space. The cardiac samples were taken through the fourth or fifth left anterior intercostal space.
Tissue Preparation
The tissues were prepared by the formalin-fixed paraffin-embedded (FFPE) method and were cut into 10-μM sections using a microtome device. They were fixed on a slide and stained using hematoxylin and eosin dyes. Two expert pathologists studied each slide. The physicians and pathologists reviewed the histopathological findings and clinical data in three joint sessions.
Results
Clinical and Laboratory Findings
We included 30 deceased patients, including 23 men and 7 women. The mean age of the patients was 73.8 ± 13.4 years, and the mean body mass index (BMI) was 27.3 ± 3.1 kg/m2. The most common underlying diseases was HTN in 25 (83.3%), IHD in 21 (70%), DM in 13 (43.3%), CRF in 10 (33.3%), and CLD in 2 (6.7%). The most common symptoms of the patients were dyspnea in 27 (90%), cough in 22 (73.3%), malaise in 20 (66.7%), fever in 14 (46.7%), and decreased level of consciousness in 12 (40%). The time between the onset of symptoms and hospital admission was 6.2 ± 2.4 days, while the time between the onset of symptoms and death was 16.4 ± 9.4 days. All patients received supportive medication comprising anticoagulation, antiacid, broad-spectrum antibiotics, and bronchodilators during their ICU admission. Also, 27 patients (90%) received dexamethasone, and 21 (70%) received remdesivir. The extension of pulmonary ground glass opacity (GGO) on CT scans was less than 40% in 11 patients (36.7%), between 40 and 60% in 8 patients (26.6%), and over 60% in 11 patients (36.7%). Three patients (10%) developed vascular complications, consisting of deep vein thrombosis in one and ecchymosis in two. The most common clinical causes of death included respiratory distress due to viral pneumonia in 13 (43.3%), sepsis and multi-organ failure in 12 (40%), pulmonary embolism in 4 (13.3%), and myocarditis in one (3.3%). Table 1 summarizes the demographic characteristics, clinical and laboratory findings, and probable clinical causes of death in our patients.
Table 1.
Main Clinical Data of the Study Population
|
Case
|
Age
|
Gender
|
BMI
(kg/m2
)
|
PMH
|
Main Symptoms
|
First SpO2
|
GGO on Lung CT
|
Days to Admission
|
Days to Death
|
WBC
(×103
U/L)
|
Hb
(g/dL)
|
PLT
(×103
U/L)
|
CRP
(mg/L)
|
D-Dimer
(<200 ng/mL)
|
LDH
(<450 U/L)
|
Trop
(<34 ng/L)
|
Clinical Cause of Death
|
| 1 |
77 |
M |
27 |
HTN, IHD |
Cough, dyspnea, malaise, LOC |
85% |
50-60% |
5 |
10 |
9.5 |
11.9 |
90 |
23 |
210 |
340 |
12 |
ARDS |
| 2 |
93 |
M |
24 |
- |
Fever, cough, dyspnea, malaise |
80% |
60-70% |
9 |
12 |
17.7 |
13.8 |
162 |
68 |
560 |
693 |
15 |
ARDS |
| 3 |
90 |
M |
26 |
HTN, IHD, CRF |
Cough, dyspnea, malaise, LOC |
78% |
60-70% |
7 |
13 |
9.6 |
9.6 |
237 |
58 |
360 |
881 |
38 |
ARDS |
| 4 |
83 |
M |
25 |
HTN, IHD |
Fever, cough, dyspnea, malaise |
85% |
70-80% |
10 |
26 |
7.7 |
11 |
187 |
48 |
457 |
567 |
411 |
Sepsis |
| 5 |
56 |
F |
30 |
HTN |
Dyspnea, malaise |
78% |
50-60% |
8 |
16 |
2 |
12.4 |
110 |
5 |
8000 |
38 |
27 |
PTE |
| 6 |
76 |
M |
26 |
HTN, IHD, DM |
Dyspnea, malaise, LOC |
88% |
40-50% |
7 |
24 |
13 |
11 |
182 |
54 |
360 |
320 |
56 |
Sepsis |
| 7 |
57 |
M |
31 |
HTN, DM |
Fever, cough, dyspnea, malaise |
90% |
70-80% |
7 |
57 |
4.3 |
14 |
186 |
38 |
120 |
441 |
34 |
Sepsis |
| 8 |
78 |
F |
24 |
HTN, CRF |
Cough, dyspnea, malaise |
86% |
30-40% |
2 |
7 |
9.7 |
14.9 |
143 |
92 |
7480 |
765 |
785 |
ARDS |
| 9 |
79 |
F |
29 |
HTN, IHD, DM |
Fever, dyspnea, LOC |
92% |
30-40% |
7 |
12 |
8 |
12.1 |
317 |
2 |
230 |
310 |
14 |
ARDS |
| 10 |
86 |
M |
26 |
HTN, IHD, DM |
Fever, LOC |
88% |
30-40% |
6 |
14 |
2.4 |
12.4 |
83 |
58 |
880 |
550 |
11 |
PTE |
| 11 |
79 |
F |
28 |
HTN, IHD |
Fever, cough, dyspnea, malaise, LOC |
91% |
20-30% |
5 |
26 |
5.9 |
13 |
130 |
11 |
380 |
281 |
31 |
Sepsis |
| 12 |
82 |
M |
24 |
HTN, IHD |
Fever, LOC |
95% |
10-20% |
6 |
13 |
14.6 |
15 |
75 |
28 |
178 |
234 |
22 |
Sepsis |
| 13 |
70 |
F |
27 |
HTN |
Fever, cough, dyspnea, malaise |
75% |
70-80% |
9 |
17 |
3.7 |
14.4 |
136 |
65 |
1200 |
753 |
47 |
PTE |
| 14 |
35 |
M |
35 |
HTN, IHD, DM |
Fever, cough, dyspnea, malaise |
75% |
70-80% |
3 |
8 |
19.1 |
11.4 |
321 |
64 |
269 |
844 |
40 |
ARDS |
| 15 |
64 |
M |
31 |
DM |
Cough, dyspnea, malaise |
84% |
70-80% |
7 |
21 |
5.9 |
13.6 |
173 |
94 |
430 |
499 |
1590 |
Myocarditis |
| 16 |
79 |
M |
25 |
HTN, IHD, DM |
Dyspnea, malaise, LOC |
81% |
30-40% |
2 |
17 |
4.1 |
13.6 |
114 |
32 |
760 |
726 |
21 |
Sepsis |
| 17 |
80 |
M |
27 |
HTN, CRF |
Fever, dyspnea, malaise, LOC |
86% |
40-50% |
8 |
17 |
7 |
13.2 |
158 |
56 |
420 |
686 |
22 |
Sepsis |
| 18 |
79 |
M |
31 |
HTN, IHD |
Fever, cough, dyspnea, malaise |
77% |
70-80% |
4 |
10 |
5.5 |
14.8 |
115 |
43 |
496 |
1573 |
11 |
ARDS |
| 19 |
55 |
M |
29 |
DM |
Cough, dyspnea |
94% |
30-40% |
7 |
19 |
3.9 |
14.4 |
102 |
24 |
370 |
310 |
30 |
Sepsis |
| 20 |
77 |
M |
26 |
HTN, IHD |
Cough, dyspnea, malaise |
94% |
30-40% |
7 |
10 |
12.4 |
13.7 |
280 |
45 |
340 |
430 |
0 |
ARDS |
| 21 |
75 |
M |
24 |
HTN, IHD, CLD |
Cough, dyspnea |
80% |
20-30% |
7 |
17 |
6.3 |
12.7 |
190 |
38 |
248 |
614 |
21 |
Sepsis |
| 22 |
91 |
M |
27 |
IHD, CLD, CRF |
Cough, dyspnea, malaise, LOC |
88% |
20-30% |
5 |
17 |
12 |
9.1 |
288 |
71 |
1312 |
600 |
18 |
Sepsis |
| 23 |
69 |
M |
28 |
HTN, IHD, DM |
Dyspnea, malaise, LOC |
80% |
60-70% |
5 |
10 |
25 |
9.2 |
397 |
66 |
430 |
476 |
57 |
ARDS |
| 24 |
70 |
M |
22 |
HTN, IHD, DM, CRF |
Cough, malaise |
92% |
40-50% |
12 |
16 |
11.4 |
13.9 |
126 |
72 |
170 |
365 |
45 |
Sepsis |
| 25 |
94 |
M |
26 |
HTN, IHD, CRF |
Cough, dyspnea |
94% |
20-30% |
10 |
30 |
7.8 |
9.8 |
235 |
54 |
250 |
232 |
21 |
Sepsis |
| 26 |
73 |
F |
31 |
HTN, IHD, DM, CRF |
Cough, dyspnea, malaise |
72% |
60-70% |
3 |
15 |
16.3 |
13.4 |
333 |
73 |
2750 |
814 |
32 |
PTE |
| 27 |
48 |
M |
32 |
HTN, IHD, CRF |
Fever, cough, dyspnea |
75% |
80-90% |
5 |
12 |
13 |
13.6 |
178 |
78 |
890 |
650 |
41 |
ARDS |
| 28 |
76 |
F |
28 |
DM, CRF |
Fever, cough, dyspnea |
82% |
50-60% |
4 |
15 |
8.9 |
11.8 |
213 |
54 |
320 |
430 |
22 |
ARDS |
| 29 |
78 |
M |
21 |
HTN, CRF |
Cough, dyspnea, LOC |
73% |
50-60% |
7 |
12 |
14 |
12.7 |
212 |
54 |
650 |
280 |
12 |
ARDS |
| 30 |
64 |
M |
28 |
HTN, IHD, DM |
Fever, cough, dyspnea |
86% |
50-60% |
3 |
13 |
5.8 |
13.8 |
137 |
46 |
540 |
827 |
60 |
ARDS |
CT, computed tomography; M, Male; F, Female; GGO, ground glass opacity; HTN, hypertension; IHD, ischemic heart disease; DM, diabetes mellitus; CRF, chronic renal failure; LOC, loss of consciousness; CLD, chronic lung disease.
Histopathological Findings
We obtained pulmonary and cardiac tissue from each patient. Three cardiac and two pulmonary tissues failed during the sampling procedure and tissue preparations. We studied 55 tissue samples, including 28 pulmonary and 27 cardiac specimens.
Pulmonary Tissue
We evaluated 28 samples of pulmonary tissues. Histopathological study revealed acute exudative phase in 5 (17.9%), sub-acute proliferative phase in 16 (57.1%), and late fibrotic phase in 7 patients (25%). The most common histopathologic findings in the pulmonary tissue included DAD in 28 (100%), hyaline membrane formation in 26 (92.8%), bronchopneumonia in 20 (71%), increased number of types II pneumocytes in 14 (50%), alveolar hemorrhage in 12 (42.8%), anthracosis in 11 (39.2%), and micro-thrombosis in one (4%). DAD comprised the exudative phase in 5 (17.9%), the exudative-proliferative phase in 16 (57.1%), and the fibrotic-organizing phase in 7 samples (25%) (Figure 1, Figure S2 and Figure S3).
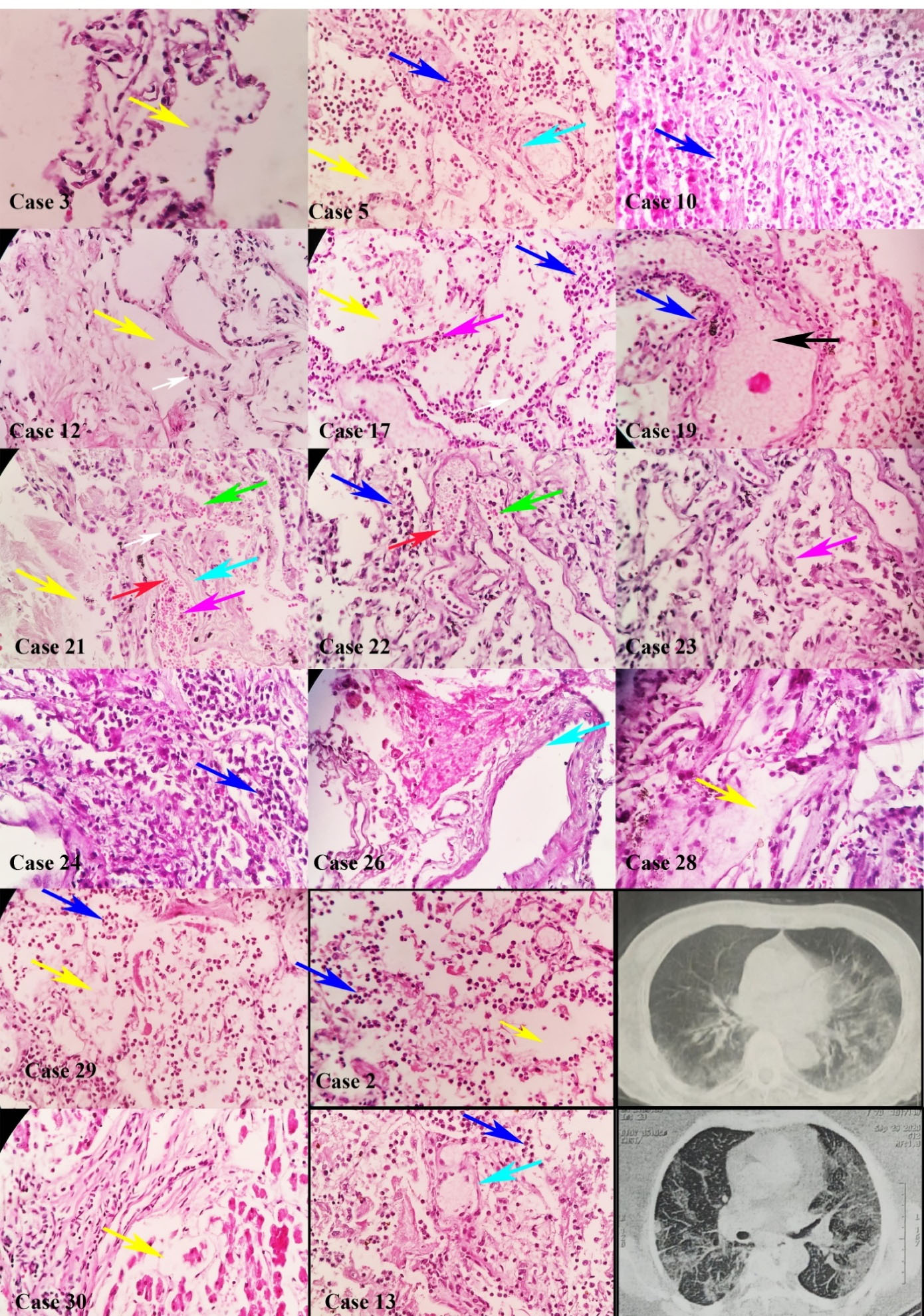
Figure 1.
Diffuse Alveolar Damage in Pulmonary Tissues of Deceased Patients with COVID-19. Pulmonary tissues in cases 2, 3, 5, 10, 12, 13, 17, 19, 21, 22, 23, 24, 26, 28, 29, and 30 showed diffuse alveolar damage in all tissues, consisting of edema (yellow arrow), intra-alveolar hemorrhage (red arrow), neutrophil clusters (blue arrow), plasma cells (white arrow), type II pneumocyte (magenta arrow), hyaline formation (cyan arrow), and fluid accumulation (black arrow) with hematoxylin and eosin dyes. Computed tomography in cases 2 and 13 (× 100 magnification)
.
Diffuse Alveolar Damage in Pulmonary Tissues of Deceased Patients with COVID-19. Pulmonary tissues in cases 2, 3, 5, 10, 12, 13, 17, 19, 21, 22, 23, 24, 26, 28, 29, and 30 showed diffuse alveolar damage in all tissues, consisting of edema (yellow arrow), intra-alveolar hemorrhage (red arrow), neutrophil clusters (blue arrow), plasma cells (white arrow), type II pneumocyte (magenta arrow), hyaline formation (cyan arrow), and fluid accumulation (black arrow) with hematoxylin and eosin dyes. Computed tomography in cases 2 and 13 (× 100 magnification)
Cardiac Tissue
We evaluated 27 samples of cardiac tissues. Histopathological evaluations showed normal tissue in 19 (70.3%), interstitial edema and myocyte hypertrophy in 7 (25.9%), and inflammatory cell infiltration compatible with myocarditis in 3 samples (11.1%) (Figure 2). Table 2 summarizes our patients’ main histopathological findings in pulmonary, cardiac, and cerebral tissues.
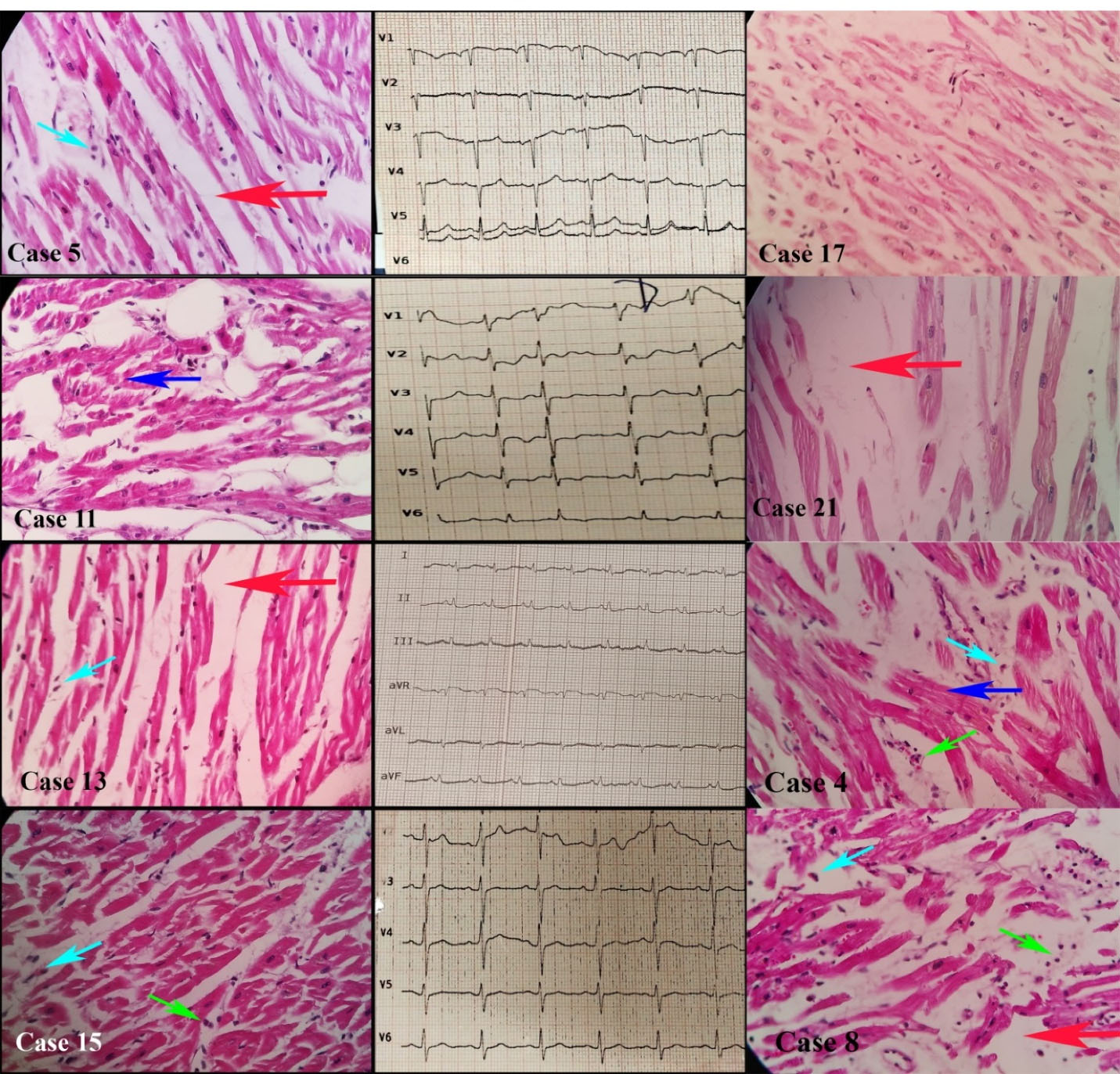
Figure 2.
Myocarditis and Myocytes Changes in Cardiac Tissues of Deceased Patients with COVID-19. Cardiac tissues in the cases 4, 5, 8, 11, 13, 15, 17, 21 showed hypertrophic myocytes (blue arrow), interstitial edema (red arrow), myocyte necrosis (cyan arrow) in the cases 4, 5, 8, 13, and 15, and mononuclear infiltration (green arrow) in cases 4, 8, and 15, with hematoxylin and eosin dyes (× 100 magnification)
.
Myocarditis and Myocytes Changes in Cardiac Tissues of Deceased Patients with COVID-19. Cardiac tissues in the cases 4, 5, 8, 11, 13, 15, 17, 21 showed hypertrophic myocytes (blue arrow), interstitial edema (red arrow), myocyte necrosis (cyan arrow) in the cases 4, 5, 8, 13, and 15, and mononuclear infiltration (green arrow) in cases 4, 8, and 15, with hematoxylin and eosin dyes (× 100 magnification)
Table 2.
Pulmonary Lesions and Cardiac Lesions
|
Case
|
Pulmonary
|
Cardiac
|
| 1 |
Hyaline membrane, exudative DAD, hemorrhage, appearance of pneumocytes II, anthracosis |
Normal |
| 2 |
Hyaline membrane, exudative and proliferative DAD, hemorrhage, appearance of pneumocytes II, bronchopneumonia |
Normal |
| 3 |
Exudative and proliferative DAD |
Normal |
| 4 |
Hyaline membrane, organizing DAD, bronchopneumonia, anthracosis |
Inflammatory infiltrations, hypertrophic myocytes and necrosis |
| 5 |
Hyaline membrane, exudative and proliferative DAD, bronchopneumonia |
Hypertrophic myocytes, interstitial edema |
| 6 |
Hyaline membrane, organizing DAD, bronchopneumonia, hemorrhage, anthracosis |
Normal |
| 7 |
Hyaline membrane, organizing DAD, bronchopneumonia, anthracosis |
Normal |
| 8 |
Hyaline membrane, exudative DAD, appearance of pneumocytes II, anthracosis |
Inflammatory infiltrations, hypertrophic myocytes and necrosis |
| 9 |
Hyaline membrane, exudative DAD, hemorrhage, appearance of pneumocytes II |
Missing Sample |
| 10 |
Hyaline membrane, exudative and proliferative DAD, hemorrhage, appearance of pneumocytes II, bronchopneumonia |
Normal |
| 11 |
Hyaline membrane, organizing DAD, bronchopneumonia, anthracosis |
Hypertrophic myocytes, interstitial edema |
| 12 |
Hyaline membrane, exudative and proliferative DAD, appearance of pneumocytes II, bronchopneumonia |
Normal |
| 13 |
Hyaline membrane, exudative and proliferative DAD, appearance of pneumocytes II, bronchopneumonia, anthracosis |
Hypertrophic myocytes, interstitial edema |
| 14 |
Hyaline membrane, exudative DAD, appearance of pneumocytes II, |
Normal |
| 15 |
Hyaline membrane, organizing DAD, bronchopneumonia |
Inflammatory infiltrations, myocytes necrosis, hemorrhage |
| 16 |
Hyaline membrane, organizing DAD, bronchopneumonia |
Normal |
| 17 |
Exudative and proliferative DAD, appearance of pneumocytes II, bronchopneumonia |
Hypertrophic myocytes, interstitial edema |
| 18 |
Missing sample |
Normal |
| 19 |
Hyaline membrane, exudative and proliferative DAD, hemorrhage, bronchopneumonia |
Missing sample |
| 20 |
Hyaline membrane, exudative DAD, appearance of pneumocytes II |
Normal |
| 21 |
Hyaline membrane, exudative and proliferative DAD, appearance of pneumocytes II, bronchopneumonia |
Hypertrophic myocytes, interstitial edema |
| 22 |
Hyaline membrane, exudative and proliferative DAD, hemorrhage, appearance of pneumocytes II, bronchopneumonia, anthracosis |
Normal |
| 23 |
Hyaline membrane, exudative and proliferative DAD, appearance of pneumocytes II, anthracosis |
Normal |
| 24 |
Hyaline membrane, exudative and proliferative DAD, hemorrhage, appearance of pneumocytes II, bronchopneumonia |
Normal |
| 25 |
Hyaline membrane, organizing DAD, hemorrhage, bronchopneumonia, anthracosis |
Missing sample |
| 26 |
Hyaline membrane, exudative and proliferative DAD, micro thrombosis, hemorrhage |
Normal |
| 27 |
Missing sample |
Normal |
| 28 |
Hyaline membrane, exudative and proliferative DAD, hemorrhage, bronchopneumonia, anthracosis |
Normal |
| 29 |
Hyaline membrane, exudative and proliferative DAD, hemorrhage, bronchopneumonia |
Normal |
| 30 |
Hyaline membrane, exudative and proliferative DAD, bronchopneumonia |
Normal |
DAD, diffuse alveolar damage.
In univariate analysis by Fisher’s exact test, we did not find any association between the clinical cause of death and troponin level (P = 0.470), C-reactive protein (CRP) level (P = 0.750), GGO extension in lung CT scan (P = 0.392), and BMI (P = 0.357). Only death due to pulmonary embolism was significantly associated with a 4-fold increase in D-dimer level (P = 0.011). Also, we did not find any association between the DAD staging and GGO extension (P = 0.702), D-dimer level (P = 0.268), CRP level (P = 0.211), and BMI (P = 0.558). Regarding cardiac pathology, we found an association between myocarditis and a two-fold rising of troponin (P = 0.010) and CRP over 80 mg/dL (P = 0.008). However, we did not find any association between myocarditis and pulmonary GGO extension (P = 0.774) or BMI (P = 1.000).
Discussion
Our study is the first comprehensive multi-organ autopsy study with a generous sample size of patients with COVID-19 in Iran. Our findings revealed that all pulmonary tissues developed DAD, while 11% of the cardiac tissue samples showed inflammation. Other findings were cardiomyocyte hypertrophy with interstitial edema in the cardiac tissues. DAD occurred most often in the proliferative and organizing phases, suggesting that most patients expired during the late stage of the disease. Previous studies reported DAD in various stages, most often in the early phase of the disease. A study in Spain on 18 deceased patients with COVID-19, with a mean age of 61 years, reported that the proliferative phase of DAD occurred in 16 patients (89%), and micro-thrombosis occurred in 6 patients (33%).9 Another study in Austria on 14 deceased patients with a mean age of 81 years showed that the organizing phase of DAD occurred in 13 (93%), and the exudative phase occurred in 12 patients (86%). In that study, all patients developed pulmonary hemorrhage, and 11 (79%) developed bronchopneumonia and micro-thrombosis.10 One study in Germany on 10 deceased patients showed an acute phase of DAD in 9 (90%) and an organizing phase in one (10%).11 We also found anthracosis in 11 of 28 pulmonary tissues (39%). A study on eight deceased patients in the United States reported anthracosis in all samples.12 Other studies did not report anthracosis, and the relationship between this finding and the severity or prognosis of COVID-19 continues to be investigated.
Earlier studies showed that numerous patients with COVID-19 have a mild elevation of D-dimer levels, but a D-dimer level higher than 2590 ng/mL was reported to be a significant risk of thromboembolism.13 In our study, 3 out of 30 patients (10%) had D-dimer levels higher than 2590 ng/mL, and only one of the 28 pulmonary tissues (3.5%) revealed thrombosis. The frequency of thrombosis in our pulmonary tissue samples was much lower than other reports. One study on 76 deceased patients in Hamburg showed that 32 patients (40%) developed deep vein thrombosis, and 17 patients (21%) developed pulmonary artery embolism.14 Another multicenter study on 68 pulmonary tissues of deceased patients with COVID-19 in the United States and Italy showed that 59 patients (87%) developed DAD, 57 patients (84%) developed micro-thrombosis, and 29 patients (43%) developed large-vessel thrombosis.15 A study by Carsana et al on 38 deceased patients with COVID-19 in Italy reported that all patients developed a 10-fold rise in D-dimer levels, and 33 (87%) developed thrombosis.16 Other studies have also reported a high prevalence of deep vein thrombosis and micro-thrombosis in deceased patients with COVID-19.17-20 This discrepancy may be due to differences in sampling techniques, study populations, and anticoagulant treatments. We used minimally invasive tissue sampling methods, which may underestimate the existing pathology.
Cardiac complications are a leading cause of death in some patients with COVID-19. They may arise from exacerbation of a pre-existing cardiac disease or an acute condition during the illness, such as infarction, thrombosis or myocarditis. In our study, 7 out of 27 (25.9%) cardiac tissues showed cardiomyocyte hypertrophy, and 3 out of 27 (11.1%) samples showed myocarditis. The frequency of myocarditis in COVID-19 varies in different studies. A study on 22 cardiac samples of deceased patients with COVID-19 with a mean age of 68 years in the United States showed a mild increase of troponin in 20 patients (90.9%), but no case of myocarditis was reported.21 Another multicenter study in the United States and Italy reported myocarditis in 3 out of 21 patients (14.2%).22 A survey of nine deceased patients with a mean age of 72 years reported only one case of myocarditis (11.1%).23 One study on four deceased patients revealed the initial stages of myocarditis in all cardiac tissue samples.24
Limited multi-organ studies were also performed on deceased patients with COVID-19. A study in South Africa on 75 deceased patients with a mean age of 60 years indicated the exudative phase of DAD in 47 (62.6%) and myocarditis in 7 (9.3%) patients.25 Another study on 21 deceased patients in Switzerland showed the exudative phase of DAD in 16 patients (76.2%), but no case of myocarditis was reported in cardiac tissues.26 A study on 11 patients in Austria showed that only one patient (9%) had myocarditis.27 Two additional multi-organ studies on deceased patients, including 21 deceased patients in the Netherlands and 10 deceased patients in the United Kingdom, showed myocarditis in 55% of the Dutch and 0% of the UK patients.28,29 Other studies on 17 and 12 deceased patients in the United States showed myocarditis in 0% and 7%, respectively.30,31 A multi-organ study on 22 deceased patients with a mean age of 68 years in Italy showed that 12 patients (54.5%) had myocarditis.32 Furthermore, a survey of 32 deceased patients with a mean age of 68 years demonstrated myocarditis in only one patient (3%).33 We did not find any similarity between our findings and other studies about myocarditis. In our research, myocarditis did not have a significant association with the severity of the disease. So, it may be attributed to other risk factors, such as viral tropism or race. We were unsure that all the histopathological findings were related to COVID-19, especially pulmonary anthracosis, increased type 2 pneumocytes, cardiac myocytes hypertrophy, and edema. Like the previous studies, our most common findings in the pulmonary tissues were DAD and hyaline membrane formation. The authors believe that these findings are related to COVID-19. However, other nonspecific findings such as hemorrhage, anthracosis, and the appearance of type II pneumocytes may be seen in other patients with underlying pulmonary diseases. In the cardiac tissues, the authors also believe that mononuclear infiltration in the patients with elevated cardiac troponin are related to COVID-19, as myocarditis. Nevertheless, other findings, such as myocyte hypertrophy and interstitial edema, are unspecific. So, we tried to avoid any complex analysis and only expressed our observations. Nevertheless, in contrast to previous studies, we found a lower frequency of micro-thrombosis in our pulmonary samples.
Conclusion
The most common pulmonary histological findings in our study was diffuse alveolar damage, similar to previous studies. Still, in cardiac tissues, we found a higher frequency of myocarditis and cardiac myocyte degeneration than in the earlier studies.
Limitations
The sampling technique was our major limitation Percutaneous needle biopsy provides less histopathological information compared to open biopsy and may underestimate pathological changes, such as thrombosis. Another limitation was the absence of a control group to compare our pathology findings between the COVID and non-COVID patients. Unavailability of immunohistochemical staining was also a limitation in differentiating inflammatory cells and immunological assays of the tissue samples.
Supplementary Files
Supplementary file 1 contains Figures S1-S3.
(pdf)
Acknowledgements
We thank our eminent colleagues, Mostafa Maddah (MD), Mohammad Afshar-Ardalan (MD), Amirhossein Shamekh (MD), and Susan Azizmohammadi (MD), for providing the requirements of this study.
Competing Interests
None.
Ethical Approval
AJA University of Medical Sciences ethics committee approved this study with the ethical approval code of IR.AJAUMS.REC.1399.030.
Funding
AJA University of Medical Sciences funded this study.
References
- Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 2022; 23(1):3-20. doi: 10.1038/s41580-021-00418-x [Crossref] [ Google Scholar]
- Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020; 55(5):2000547. doi: 10.1183/13993003.00547-2020 [Crossref] [ Google Scholar]
- Fang X, Li S, Yu H, Wang P, Zhang Y, Chen Z. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020; 12(13):12493-503. doi: 10.18632/aging.103579 [Crossref] [ Google Scholar]
- Konopka KE, Nguyen T, Jentzen JM, Rayes O, Schmidt CJ, Wilson AM. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 infection is morphologically indistinguishable from other causes of DAD. Histopathology 2020; 77(4):570-8. doi: 10.1111/his.14180 [Crossref] [ Google Scholar]
- Craver R, Huber S, Sandomirsky M, McKenna D, Schieffelin J, Finger L. Fatal eosinophilic myocarditis in a healthy 17-year-old male with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2c). Fetal Pediatr Pathol 2020; 39(3):263-8. doi: 10.1080/15513815.2020.1761491 [Crossref] [ Google Scholar]
- Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection 2020; 48(5):773-7. doi: 10.1007/s15010-020-01424-5 [Crossref] [ Google Scholar]
- Jensen MP, Le Quesne J, Officer-Jones L, Teodòsio A, Thaventhiran J, Ficken C. Neuropathological findings in two patients with fatal COVID-19. Neuropathol Appl Neurobiol 2021; 47(1):17-25. doi: 10.1111/nan.12662 [Crossref] [ Google Scholar]
- Hirschbühl K, Schaller T, Kling E, Märkl B, Claus R. Autopsy of patients with COVID-19: a balance of fear and curiosity. Pathol Res Pract 2020; 216(8):153039. doi: 10.1016/j.prp.2020.153039 [Crossref] [ Google Scholar]
- Valdivia-Mazeyra MF, Salas C, Nieves-Alonso JM, Martín-Fragueiro L, Bárcena C, Muñoz-Hernández P. Increased number of pulmonary megakaryocytes in COVID-19 patients with diffuse alveolar damage: an autopsy study with clinical correlation and review of the literature. Virchows Arch 2021; 478(3):487-96. doi: 10.1007/s00428-020-02926-1 [Crossref] [ Google Scholar]
- Grosse C, Grosse A, Salzer HJF, Dünser MW, Motz R, Langer R. Analysis of cardiopulmonary findings in COVID-19 fatalities: high incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol 2020; 49:107263. doi: 10.1016/j.carpath.2020.107263 [Crossref] [ Google Scholar]
- Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B. Postmortem examination of patients with COVID-19. JAMA 2020; 323(24):2518-20. doi: 10.1001/jama.2020.8907 [Crossref] [ Google Scholar]
- Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis 2020; 26(9):2005-15. doi: 10.3201/eid2609.202095 [Crossref] [ Google Scholar]
- Mouhat B, Besutti M, Bouiller K, Grillet F, Monnin C, Ecarnot F. Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. Eur Respir J 2020; 56(4):2001811. doi: 10.1183/13993003.01811-2020 [Crossref] [ Google Scholar]
- Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med 2020; 134(4):1275-84. doi: 10.1007/s00414-020-02317-w [Crossref] [ Google Scholar]
- Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, Mostyka M. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol 2020; 33(11):2156-68. doi: 10.1038/s41379-020-00661-1 [Crossref] [ Google Scholar]
- Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020; 20(10):1135-40. doi: 10.1016/s1473-3099(20)30434-5 [Crossref] [ Google Scholar]
- Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020; 173(4):268-77. doi: 10.7326/m20-2003 [Crossref] [ Google Scholar]
- Damiani S, Fiorentino M, De Palma A, Foschini MP, Lazzarotto T, Gabrielli L. Pathological post-mortem findings in lungs infected with SARS-CoV-2. J Pathol 2021; 253(1):31-40. doi: 10.1002/path.5549 [Crossref] [ Google Scholar]
- Kommoss FKF, Schwab C, Tavernar L, Schreck J, Wagner WL, Merle U. The pathology of severe COVID-19-related lung damage. Dtsch Arztebl Int 2020; 117(29-30):500-6. doi: 10.3238/arztebl.2020.0500 [Crossref] [ Google Scholar]
- Sadegh Beigee F, Pourabdollah Toutkaboni M, Khalili N, Nadji SA, Dorudinia A, Rezaei M. Diffuse alveolar damage and thrombotic microangiopathy are the main histopathological findings in lung tissue biopsy samples of COVID-19 patients. Pathol Res Pract 2020; 216(10):153228. doi: 10.1016/j.prp.2020.153228 [Crossref] [ Google Scholar]
- Fox SE, Li G, Akmatbekov A, Harbert JL, Lameira FS, Brown JQ. Unexpected features of cardiac pathology in COVID-19 infection. Circulation 2020; 142(11):1123-5. doi: 10.1161/circulationaha.120.049465 [Crossref] [ Google Scholar]
- Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 2020; 41(39):3827-35. doi: 10.1093/eurheartj/ehaa664 [Crossref] [ Google Scholar]
- Youd E, Moore L. COVID-19 autopsy in people who died in community settings: the first series. J Clin Pathol 2020; 73(12):840-4. doi: 10.1136/jclinpath-2020-206710 [Crossref] [ Google Scholar]
- Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 2020; 8(7):681-6. doi: 10.1016/s2213-2600(20)30243-5 [Crossref] [ Google Scholar]
- Nunes MC, Hale MJ, Mahtab S, Mabena FC, Dludlu N, Baillie VL. Clinical characteristics and histopathology of COVID-19 related deaths in South African adults. PLoS One 2022; 17(1):e0262179. doi: 10.1371/journal.pone.0262179 [Crossref] [ Google Scholar]
- Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020; 77(2):198-209. doi: 10.1111/his.14134 [Crossref] [ Google Scholar]
- Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med 2020; 173(5):350-61. doi: 10.7326/m20-2566 [Crossref] [ Google Scholar]
- Schurink B, Roos E, Radonic T, Barbe E, Bouman CSC, de Boer HH. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe 2020; 1(7):e290-e9. doi: 10.1016/s2666-5247(20)30144-0 [Crossref] [ Google Scholar]
- Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe 2020; 1(6):e245-e53. doi: 10.1016/s2666-5247(20)30115-4 [Crossref] [ Google Scholar]
- Remmelink M, De Mendonça R, D’Haene N, De Clercq S, Verocq C, Lebrun L. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care 2020; 24(1):495. doi: 10.1186/s13054-020-03218-5 [Crossref] [ Google Scholar]
- Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 2020; 396(10247):320-32. doi: 10.1016/s0140-6736(20)31305-2 [Crossref] [ Google Scholar]
- Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis 2020; 222(11):1807-15. doi: 10.1093/infdis/jiaa578 [Crossref] [ Google Scholar]
- Elsoukkary SS, Mostyka M, Dillard A, Berman DR, Ma LX, Chadburn A. Autopsy findings in 32 patients with COVID-19: a single-institution experience. Pathobiology 2021; 88(1):56-68. doi: 10.1159/000511325 [Crossref] [ Google Scholar]