Arch Iran Med. 26(10):575-581.
doi: 10.34172/aim.2023.84
Original Article
Translation, Cultural Adaptation, Validation and Reliability of Persian Left Ventricular Dysfunction–36 Questionnaire
Mehrbod Vakhshoori Conceptualization, Formal analysis, Supervision, Writing – original draft, Writing – review & editing, 1, # 
Niloofar Bondariyan Conceptualization, Writing – original draft, Writing – review & editing, 2, #
Sayed Ali Emami Conceptualization, Data curation, Writing – original draft, Writing – review & editing, 1
Niyousha Sadeghpour Data curation, Writing – original draft, Writing – review & editing, 1
Farbod Khanizadeh Formal analysis, Supervision, Writing – original draft, 3
Mahmood Emami Supervision, Writing – original draft, 4
Davood Shafie Conceptualization, Formal analysis, Supervision, Writing – original draft, Writing – review & editing, 1, * 
Author information:
1Heart Failure Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
2Department of Clinical Pharmacy, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
3Insurance Research Center, Tehran, Iran
4Yazd Cardiovascular Research Institute, Yazd University of Medical Sciences, Yazd, Iran
#Mehrbod Vakhshoori and Niloofar Bondariyan contributed equally as first authors.
Abstract
Background:
The left ventricular dysfunction 36 (LVD-36) questionnaire is considered to be a tool to assess the impact of left ventricle impairment on patients’ daily life. This methodological study was aimed to translate and assess the validity and reliability of the Persian draft of the LVD-36 questionnaire among Iranian heart failure (HF) patients.
Methods:
We recruited stable HF patients who referred to an outpatient heart clinic in Isfahan, Iran. The LVD-36 questionnaire was translated using the forward-backward method. Twenty HF patients were recruited for content validity assessment and were asked to express their opinions about the comprehensibility and meaningfulness of each item. We invited 14 experts to assess validity through content validity index (CVI) and content validity ratio (CVR). Reliability was assessed by Cronbach’s alpha and intraclass correlation coefficient (ICC), with the latter evaluated after invitation of the participants to complete the questionnaire for the second time.
Results:
The translation process was performed uneventfully without any significant alterations. A total of 150 HF patients were recruited to assess the reliability of the questionnaire in this study (age: 64.6±16 years, males: 58.6%). All items had acceptable CVI and CVR, ranging 0.85–1.00 and 0.57–1.00, respectively. Cronbach’s alpha was 0.971. All participants completed the questionnaire for the second time with no missing data. Test-retest reliability revealed an excellent ICC value of 0.981 (95% CI: 0.977–0.985).
Conclusion:
The Persian version of the LVD-36 questionnaire is a simple, valid and reliable tool for evaluating the impact of left ventricle impairment on the well-being of Iranian HF patients.
Keywords: Heart failure, Iran, Left ventricular dysfunction, Quality of life, Reproducibility of results, Validation study
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Vakhshoori M, Bondariyan N, Emami SA, Sadeghpour N, Khanizadeh F, Emami M, et al. Translation, cultural adaptation, validation and reliability of persian left ventricular dysfunction–36 questionnaire. Arch Iran Med. 2023;26(10):575- 581. doi: 10.34172/aim.2023.84
Introduction
Heart failure (HF) is defined as impaired ventricular filling or decreased heart ability to pump blood to meet systemic needs. It is divided into right and left HF with different signs and symptoms. Left ventricle dysfunction (LVD) is the most common cause of congestive HF in the context of several etiologies including hypertension, ischemic heart disease, diabetes, and structural problems of the heart.1-3 Left HF manifestations are paroxysmal nocturnal dyspnea, shortness of breath, orthopnea, and/or symptoms of volume overload (including increased abdominal girth, weight gain, leg swelling, or right upper quadrant pain due to liver congestion)1, which are disabling and negatively impact patients’ quality of life.4 HF is also known as a major growing medical and economic problem worldwide.5,6 According to the World Health Organization (WHO), the quality of life (QoL) is defined as: “an individual’s perception of their position in life in the context of the culture and value systems in which they live and their goals, expectations, and standards, and concerns”. This concept is influenced by various factors, including physical health, personal beliefs, psychological well-being, social relationships, and the individual’s interaction with salient features of their environment.7
QoL is one of the most critical aspects of health which is affected by illnesses, and without a doubt, chronic diseases are more serious. They are increasingly becoming more and more common in recent decades.4 For patients with chronic conditions like mental disorders, asthma, kidney disease, chronic obstructive pulmonary disease, and congestive HF, evaluating the “quality of life” through health status measurement has become crucial in assessing the disease impact and effectiveness of treatment.8
Questionnaires are used as tools to measure the QoL. Due to the variety of illnesses and their consequent morbidities, several QoL questionnaires are needed for further evaluation. In terms of congestive HF, several related questionnaires are available such as Chronic Heart Failure Assessment Tool (CHAT), Cardiac Health Profile congestive heart failure (CHPchf), Chronic Heart Failure Questionnaire (CHFQ), Kansas City Cardiomyopathy Questionnaire (KCCQ), Minnesota Living with Heart Failure Questionnaire (MLHFQ) and Quality of Life Questionnaire in Severe Heart Failure (QLQ-SHF), each of which has a specific application and is sometimes used in combination with or instead of others.9-14 However, using these questionnaires might be associated with difficulties regarding the required time in order to fill out the questionnaire completely as well as the total questionnaire length. Left Ventricular Disease Questionnaire (LVDQ) is a new short questionnaire designed for patients suffering from left ventricular impairment and can be completed in a timely manner. It is composed of 36 questions with true/false answers, and the results will be presented in the range 0–100%, which are the best and the worst scores, respectively.15 This questionnaire is also known as the left ventricular dysfunction questionnaire (LVD-36) and has been reported as a reliable and suitable tool for use to assess quality of life among patients with LVD.16,17
The LVD-36 is originally in English and has been translated into other languages like Italian.18 Due to its importance and appliance and lack of a Persian version of the questionnaire, it needs to be translated into Persian for use in Iran. In this article, we decided to translate the questionnaire into Persian and examine its validity and reliability among Persian-speaking patients in Iran.
Materials and Methods
LVD-36 Questionnaire
The LVD-36 questionnaire is specifically designed to assess the impact of left ventricular impairment on the daily life of HF patients. It consists of 36 items, each with a true or false answering scale. The responses are summed, and the final score is reported as a percentage ranging from 0 (indicating the best possible score) to 100 (representing the worst possible score).15
Translation
The translation process of the questionnaire, reported by Beaton et al, involved five phases.19 First, two bilingual translators, both native Persian speakers with fluency in English, were enlisted to independently translate the questionnaire from English to Persian. Only one of the translators had familiarity with medical terminology. They were asked to use meaningful and straightforward terms for translation at the second step. The first translated version was created after a final consensus between translators. Next, this Persian draft was translated backward to English. A meeting was held to discuss the pre-final version of the questionnaire with two translators and methodologists in the fourth step. Finally, we distributed the questionnaire among 20 patients who suffered from HF. They were asked to express their understanding of each question to the principal investigator. Their comments and probable questions were reviewed for possible modification of the questionnaire.
Validity
A total of 14 experts, including 5 cardiologists, 2 general practitioners, 2 pharmacists, 1 statistician, and 4 nurses, were invited to assess the validity of the questionnaire. Each expert provided their feedback regarding the relevance, understandability, and comprehensibility of each item using a 4-point rating scale. The scale options were as follows: (1) not suitable, (2) suitable with minor adjustments, (3) suitable but requiring some modifications, and (4) very suitable. To calculate the CVI, the number of experts who rated an item as 3 or 4 was divided by the total number of experts. We considered the value of 0.80 as acceptable. Lower item CVI was defined as 0.70–0.79: re-evaluation required, < 70: candidate for omission.20,21 For CVR measurement, the experts were required to declare their opinions about each provided item on the questionnaire with a 3-item scale (essential, important but not essential, not essential). A ratio of at least 0.57 was defined as acceptable.22
Reliability
We evaluated the internal consistency of the questionnaire using Cronbach’s alpha coefficient, which is a measure of reliability. The interpretation of Cronbach’s alpha values is as follows: ≥ 0.9 indicates excellent internal consistency, 0.8–0.9 indicates good internal consistency, 0.7–0.79 indicates acceptable internal consistency, 0.6–0.69 indicates questionable internal consistency, 0.5–0.59 indicates poor internal consistency, and < 0.5 indicates unacceptable internal consistency.23 We measured test-retest reliability by asking the recruited participants to answer the questionnaire questions for the second time after approximately two weeks, and the calculated ICC was characterized as follows: ≥ 0.75: excellent, 0.4–0.75: fair to good, and < 0.4: poor.24
Floor and Ceiling Effect
We evaluated the presence of floor and ceiling effects by examining whether more than 15% of the study population obtained the lowest and highest scores, respectively.
Statistical Analysis
The distribution of the participants’ answers on each questionnaire item was reported as frequency (percentage). Also, mean ± standard deviation (SD) was used to report total scores. All analyses were done with the Statistical Package for Social Sciences (SPSS) version 22 (IBM Corp., Armonk, NY, USA).
Ethical Considerations
The Ethics Committee of Isfahan University of medical sciences (IUMS) approved this study (IR.MUI.MED.REC.1400.562). At the start of the study, the principal investigator provided a comprehensive explanation of the main objectives to each participant. Ample time was given to participants to ask any questions they had about the project. Moreover, all individuals were assured that their personal information would remain confidential and not divulged publicly. Finally, each patient was provided with a written consent form to sign.
Results
Questionnaire Performance
Out of 20 HF patients who fulfilled the questionnaire, most declared that all the questions were easily understandable in the translated version and no major changes were made in the final questionnaire. Figure 1 shows the Persian version of the LVD-36 questionnaire in comparison to the original one. The final Persian draft of the LVD-36 questionnaire is presented in Supplementary file 1.
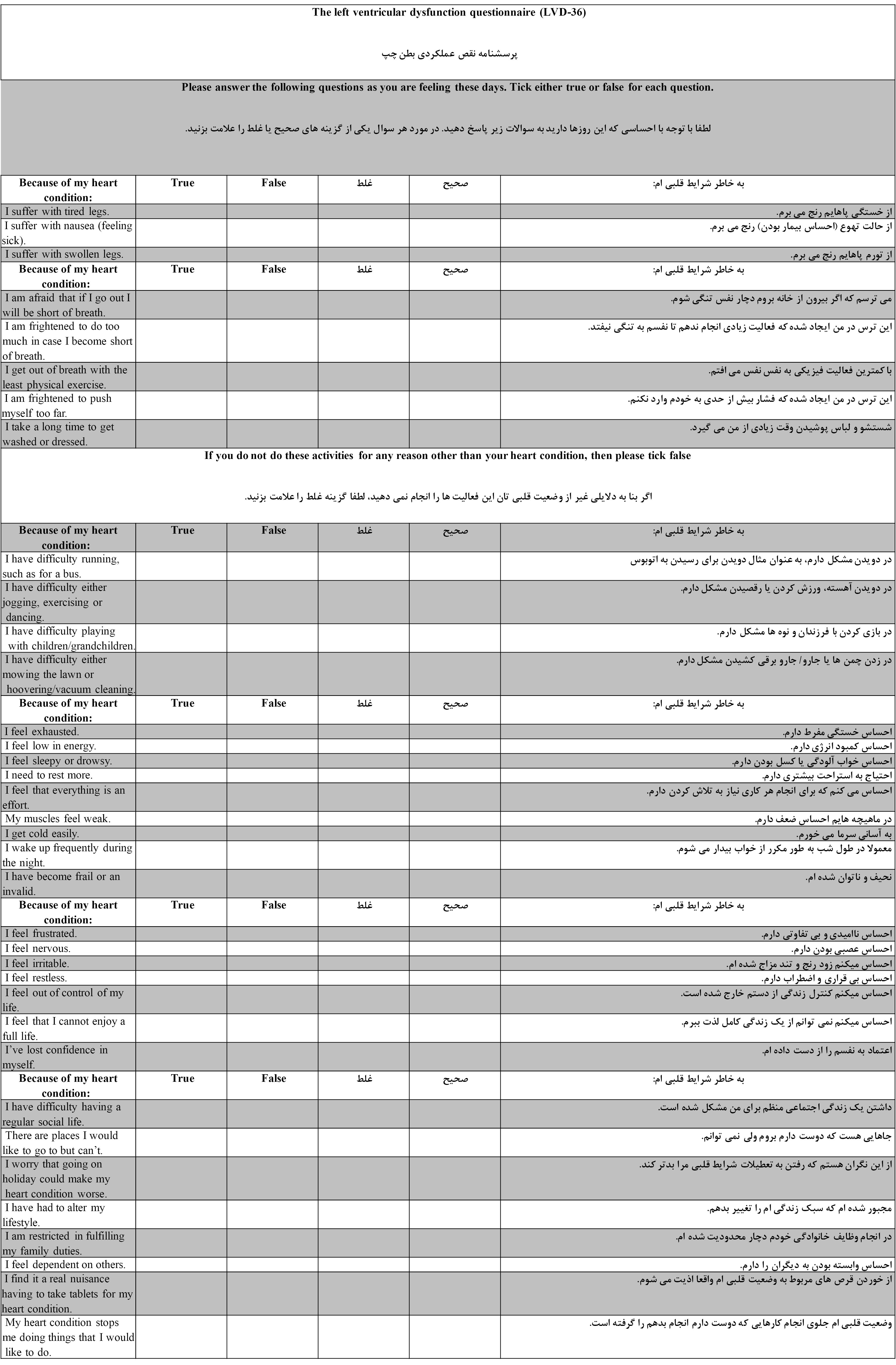
Figure 1.
Persian and Original Version of LVD-36 Questionnaire
.
Persian and Original Version of LVD-36 Questionnaire
Questionnaire Validity
Table 1 presents data on the CVI and CVR of each questionnaire item. All questions had CVI ranging from 0.85 to 1.00. Moreover, each item showed acceptable CVR (minimum: 0.57, maximum: 1.00).
Table 1.
Validity Indices of the Persian Version of the Left Ventricle Dysfunction-36 (LVD-36) Questionnaire
|
Questions
|
Content Validity Index
|
Content Validity Ratio
|
| Item 1 |
0.85 |
0.86 |
| Item 2 |
0.92 |
0.57 |
| Item 3 |
1.00 |
0.86 |
| Item 4 |
0.92 |
0.71 |
| Item 5 |
1.00 |
0.57 |
| Item 6 |
1.00 |
0.71 |
| Item 7 |
1.00 |
0.71 |
| Item 8 |
0.85 |
0.86 |
| Item 9 |
0.85 |
0.57 |
| Item 10 |
0.85 |
1.00 |
| Item 11 |
0.92 |
0.86 |
| Item 12 |
0.92 |
1.00 |
| Item 13 |
1.00 |
0.86 |
| Item 14 |
0.92 |
0.86 |
| Item 15 |
0.85 |
0.86 |
| Item 16 |
0.85 |
0.57 |
| Item 17 |
1.00 |
0.86 |
| Item 18 |
1.00 |
0.71 |
| Item 19 |
0.92 |
1.00 |
| Item 20 |
0.85 |
0.71 |
| Item 21 |
1.00 |
1.00 |
| Item 22 |
0.92 |
1.00 |
| Item 23 |
0.92 |
0.71 |
| Item 24 |
1.00 |
1.00 |
| Item 25 |
0.92 |
1.00 |
| Item 26 |
1.00 |
0.57 |
| Item 27 |
0.85 |
1.00 |
| Item 28 |
1.00 |
1.00 |
| Item 29 |
1.00 |
0.71 |
| Item 30 |
0.85 |
0.86 |
| Item 31 |
0.92 |
1.00 |
| Item 32 |
1.00 |
0.71 |
| Item 33 |
0.85 |
1.00 |
| Item 34 |
0.92 |
1.00 |
| Item 35 |
0.85 |
1.00 |
| Item 36 |
0.92 |
0.57 |
Participants’ Characteristics
A total of 150 HF patients completed the questionnaire on two separate occasions, with a two-week interval between each administration (age: 64.6 ± 16 years, males: 58.6%). The distribution of their answers based on each item on the first and second round of questionnaire completion is shown in Table 2. The mean LVD score after the first completion was 94.09 ± 16.3%.
Table 2.
Distribution of Respondents’ Answers during the First and Second Round of Completing the Persian Left Ventricle Dysfunction-36 (LVD-36) Questionnaire (n = 150)
|
Questions
|
First Round
|
Second Round
|
|
Yes (%)
|
No (%)
|
Yes (%)
|
No (%)
|
| Item 1 |
140 (93) |
10 (15) |
117 (78) |
33 (22) |
| Item 2 |
141 (93) |
9 (15) |
138 (92) |
12 (15) |
| Item 3 |
107 (71) |
43 (29) |
67 (45) |
83 (55) |
| Item 4 |
136 (91) |
14 (9) |
139 (93) |
11 (15) |
| Item 5 |
140 (93) |
10 (15) |
140 (93) |
10 (15) |
| Item 6 |
142 (95) |
8 (5) |
142 (95) |
8 (5) |
| Item 7 |
142 (95) |
8 (5) |
142 (95) |
8 (5) |
| Item 8 |
133 (89) |
17 (11) |
134 (90) |
16 (10) |
| Item 9 |
147 (98) |
3 (2) |
147 (98) |
3 (2) |
| Item 10 |
136 (91) |
14 (9) |
138 (92) |
12 (15) |
| Item 11 |
135 (90) |
15 (10) |
134 (90) |
16 (10) |
| Item 12 |
140 (93) |
10 (15) |
141 (93) |
9 (15) |
| Item 13 |
144 (96) |
6 (4) |
141 (93) |
9 (15) |
| Item 14 |
146 (97) |
4 (3) |
146 (97) |
4 (3) |
| Item 15 |
145 (97) |
5 (3) |
145 (97) |
5 (3) |
| Item 16 |
145 (97) |
5 (3) |
143 (95) |
7 (5) |
| Item 17 |
145 (97) |
5 (3) |
145 (97) |
5 (3) |
| Item 18 |
147 (98) |
3 (2) |
146 (97) |
4 (3) |
| Item 19 |
147 (98) |
3 (2) |
144 (96) |
6 (4) |
| Item 20 |
145 (97) |
5 (3) |
142 (95) |
8 (5) |
| Item 21 |
144 (96) |
6 (4) |
141 (93) |
9 (15) |
| Item 22 |
145 (97) |
5 (3) |
144 (96) |
6 (4) |
| Item 23 |
138 (92) |
12 (15) |
138 (92) |
12 (15) |
| Item 24 |
141 (93) |
9 (15) |
141 (93) |
9 (15) |
| Item 25 |
143 (95) |
7 (5) |
143 (95) |
7 (5) |
| Item 26 |
141 (93) |
9 (15) |
141 (93) |
9 (15) |
| Item 27 |
141 (93) |
9 (15) |
141 (93) |
9 (15) |
| Item 28 |
141 (93) |
9 (15) |
141 (93) |
9 (15) |
| Item 29 |
139 (93) |
11 (15) |
139 (93) |
11 (15) |
| Item 30 |
142 (95) |
8 (5) |
142 (95) |
8 (5) |
| Item 31 |
142 (95) |
8 (5) |
142 (95) |
8 (5) |
| Item 32 |
144 (96) |
6 (4) |
144 (96) |
6 (4) |
| Item 33 |
143 (95) |
7 (5) |
143 (95) |
7 (5) |
| Item 34 |
144 (96) |
6 (4) |
144 (96) |
6 (4) |
| Item 35 |
145 (97) |
5 (3) |
145 (97) |
5 (3) |
| Item 36 |
145 (97) |
5 (3) |
145 (97) |
5 (3) |
| Total score |
94.09 ± 16.3% |
92.7 ± 16.3% |
Questionnaire Reliability
The Cronbach’s alpha coefficient of the current Persian version of the LVD-36 questionnaire was 0.971, showing excellent internal consistency. The ICC of the questionnaire two weeks after the second round of completion was found to be 0.981 (95% confidence interval [CI]: 0.977-0.985). The reliability indices of questionnaire items are shown in Table 3. The minimum and maximum values of corrected item-total correlation (CITC) were 0.234 (item 18) and 0.943 (items 30 and 31), respectively. Also, all items contributed desirably to internal reliability in a way that deletion of questions did not significantly increase the value of Cronbach’s alpha.
Table 3.
Reliability Indices of the Persian Version of the Left Ventricle Dysfunction-36 (LVD-36) Questionnaire
|
Questions
|
Corrected Item-Total Correlation
|
Cronbach's Alpha If Item Deleted
|
| Item 1 |
0.621 |
0.970 |
| Item 2 |
0.789 |
0.969 |
| Item 3 |
0.387 |
0.974 |
| Item 4 |
0.674 |
0.970 |
| Item 5 |
0.727 |
0.970 |
| Item 6 |
0.753 |
0.970 |
| Item 7 |
0.775 |
0.970 |
| Item 8 |
0.748 |
0.970 |
| Item 9 |
0.625 |
0.970 |
| Item 10 |
0.662 |
0.970 |
| Item 11 |
0.614 |
0.971 |
| Item 12 |
0.640 |
0.970 |
| Item 13 |
0.535 |
0.971 |
| Item 14 |
0.696 |
0.970 |
| Item 15 |
0.690 |
0.970 |
| Item 16 |
0.690 |
0.970 |
| Item 17 |
0.637 |
0.970 |
| Item 18 |
0.234 |
0.971 |
| Item 19 |
0.483 |
0.971 |
| Item 20 |
0.677 |
0.970 |
| Item 21 |
0.748 |
0.970 |
| Item 22 |
0.499 |
0.971 |
| Item 23 |
0.831 |
0.969 |
| Item 24 |
0.672 |
0.970 |
| Item 25 |
0.740 |
0.970 |
| Item 26 |
0.902 |
0.969 |
| Item 27 |
0.902 |
0.969 |
| Item 28 |
0.713 |
0.970 |
| Item 29 |
0.884 |
0.969 |
| Item 30 |
0.943 |
0.969 |
| Item 31 |
0.943 |
0.969 |
| Item 32 |
0.784 |
0.970 |
| Item 33 |
0.883 |
0.969 |
| Item 34 |
0.852 |
0.969 |
| Item 35 |
0.750 |
0.970 |
| Item 36 |
0.717 |
0.970 |
Floor and Ceiling Effect
No instances of the highest or lowest scores were observed in our study population, indicating the absence of both floor and ceiling effects.
Discussion
In this study, we aimed to investigate the validity and reliability of the Persian version of the LVD-36 questionnaire. We found that this questionnaire could appropriately assess the main health issues of Iranian patients suffering from HF. The prevalence of HF has increased during recent years, and assessing its effect on patients’ daily life could significantly aid in better implementation of therapeutic strategies.
This questionnaire was first developed based on 179 items collected from multiple sources, including prior questionnaires, published records, patient interviews, and physician consultations. After the completion of all items by 139 individuals suffering from left ventricular impairment and further analysis, 36 items were finally selected, and the final version of the LVD-36 questionnaire was officially introduced.25,26
We used one of the most common translation methods, namely the forward-backward method. The dual-panel is another method using two different phases, including a bilingual panel and a lay panel. The first panel conducts the initial translation of the questionnaire, while the second group, consisting of monolingual individuals, is asked to provide feedback on the clarity and comprehensibility of the translated version.27,28 It has been reported that the dual-panel method has fewer missing items during the translation process. However, this method is more time-consuming and might be associated with difficulties to perform thoroughly.29 Moreover, it has been recommended that the translated draft of the questionnaire is needed to be combined with backward translation.27,30 Therefore, we decided to use the common forward-backward translation method. Also, our findings revealed that there was not any missing item during translation of the original LVD-36 questionnaire. The respondents declared that the questionnaire had appropriately understandable questions and needed approximately five minutes for completion.
Our results in terms of reliability, as an index to evaluate the ability of the questionnaire for accurate measurement, revealed a Cronbach’s alpha of 0.971. Also, after completing the questionnaire for the second time by all participants, the ICC value was found to be 0.981 (95% CI: 0.977–0.985), that indicates this questionnaire is a reliable tool for assessing general health among HF patients. O’Leary and Jones recruited 60 patients with chronic left ventricle dysfunction to assess the reliability of the LVD-36 questionnaire. All patients completed the questionnaire for the first time, with 53 subjects re-completing one to three weeks later. The mean time reported to be taken in order to complete the questionnaire was approximately 5 minutes. The internal consistency of the LVD-36 questionnaire was 0.95 with a similar ICC (0.95). They suggested that this questionnaire has a high degree of reliability with unambiguous wording.15 Moreover, Miani et al recruited 50 patients with congestive HF and translated the original questionnaire to the Italian language. After assessing reliability, they suggested that this instrument was a reliable tool among Italian patients.18 The floor and ceiling effect values in another study were 0 and 3%, respectively.15 However, we did not observe this effect among our responders.
This study demonstrates that the Persian version of the LVD-36 questionnaire showed good content validity, indicating that the instrument accurately measured its intended constructs. Additionally, a comparison between the LVD-36 and the short-form 36 (SF-36) questionnaire revealed stronger correlations in physical well-being and weaker correlations in psychosocial well-being.15 Another study revealed that the Italian version of the LVD-36 questionnaire correlated well with the MLHF questionnaire.18
To the best of our knowledge, this study is the first in the literature to assess the reliability and validity of the Persian-translated LVD-36 questionnaire among Iranian HF patients. Completing by a large number of study samples twice without any missing data could be considered another strength of the current study. However, some limitations also exist. This study was implemented in a single center, and our findings might not be generalizable to other Iranian individuals living in other Iranian cities. We also did not assess the educational levels of the enrolled participants, which might have affected our outcomes.
Conclusion
In conclusion, the Persian version of the LVD-36 questionnaire is as accurate and reliable as the original version. It is simple and understandable, has a high reliability and validity index, and can be used in clinical settings to assess the quality of life and general health status of Iranian HF patients.
Supplementary Files
Supplementary file 1 contains Figure S1.
(pdf)
Competing Interests
None of the authors had any personal or financial conflicts of interest.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to confidential issues but are available from the corresponding author on reasonable request.
Ethical Approval
All procedures performed in studies involving human participants were under the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The ethics committee affiliated to Isfahan University of Medical Sciences (IUMS) proved this study (IR.MUI.MED.REC.1400.562).
Written informed consent was obtained from the patients. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- Malik A, Brito D, Vaqar S, Chhabra L. Congestive heart failure. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023.
- Pazos-López P, Peteiro-Vázquez J, Carcía-Campos A, García-Bueno L, de Torres JP, Castro-Beiras A. The causes, consequences, and treatment of left or right heart failure. Vasc Health Risk Manag 2011; 7:237-54. doi: 10.2147/vhrm.s10669 [Crossref] [ Google Scholar]
- Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess 2009; 13(32):1-207, iii. doi: 10.3310/hta13320 [Crossref] [ Google Scholar]
- Megari K. Quality of life in chronic disease patients. Health Psychol Res 2013; 1(3):e27. doi: 10.4081/hpr.2013.e27 [Crossref] [ Google Scholar]
- Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004-2016. BMC Cardiovasc Disord 2018; 18(1):74. doi: 10.1186/s12872-018-0815-3 [Crossref] [ Google Scholar]
- Agbor VN, Ntusi NAB, Noubiap JJ. An overview of heart failure in low- and middle-income countries. Cardiovasc Diagn Ther 2020; 10(2):244-51. doi: 10.21037/cdt.2019.08.03 [Crossref] [ Google Scholar]
- WHOQOL Group. Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Qual Life Res 1993; 2(2):153-9. doi: 10.1007/bf00435734 [Crossref] [ Google Scholar]
- Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci 2011; 66(3):301-11. doi: 10.1093/gerona/glq208 [Crossref] [ Google Scholar]
- Dunderdale K, Thompson DR, Beer SF, Furze G, Miles JN. Development and validation of a patient-centered health-related quality-of-life measure: the chronic heart failure assessment tool. J Cardiovasc Nurs 2008; 23(4):364-70. doi: 10.1097/01.JCN.0000317439.82704.e8 [Crossref] [ Google Scholar]
- Mannheimer B, Andersson B, Carlsson L, Währborg P. The validation of a new quality of life questionnaire for patients with congestive heart failure-an extension of the cardiac health profile. Scand Cardiovasc J 2007; 41(4):235-41. doi: 10.1080/14017430701422454 [Crossref] [ Google Scholar]
- Guyatt GH, Nogradi S, Halcrow S, Singer J, Sullivan MJ, Fallen EL. Development and testing of a new measure of health status for clinical trials in heart failure. J Gen Intern Med 1989; 4(2):101-7. doi: 10.1007/bf02602348 [Crossref] [ Google Scholar]
- Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000; 35(5):1245-55. doi: 10.1016/s0735-1097(00)00531-3 [Crossref] [ Google Scholar]
- Rector TS, Kubo SH, Cohn JN. Patient’s self-assessment of their congestive heart failure: content, reliability, validity of a new measure, the Minnesota living with heart failure questionnaire. Heart Fail 1987; 3:198-209. [ Google Scholar]
- Wiklund I, Lindvall K, Swedberg K, Zupkis RV. Self-assessment of quality of life in severe heart failure An instrument for clinical use. Scand J Psychol 1987; 28(3):220-5. doi: 10.1111/j.1467-9450.1987.tb00758.x [Crossref] [ Google Scholar]
- O’Leary CJ, Jones PW. The left ventricular dysfunction questionnaire (LVD-36): reliability, validity, and responsiveness. Heart 2000; 83(6):634-40. doi: 10.1136/heart.83.6.634 [Crossref] [ Google Scholar]
- Psotka MA, von Maltzahn R, Anatchkova M, Agodoa I, Chau D, Malik FI. Patient-reported outcomes in chronic heart failure: applicability for regulatory approval. JACC Heart Fail 2016; 4(10):791-804. doi: 10.1016/j.jchf.2016.04.010 [Crossref] [ Google Scholar]
- Garin O, Herdman M, Vilagut G, Ferrer M, Ribera A, Rajmil L. Assessing health-related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev 2014; 19(3):359-67. doi: 10.1007/s10741-013-9394-7 [Crossref] [ Google Scholar]
- Miani D, Gregori D, Ghidina M, Rozbowsky P, Pilotto L, Albanese MC. The left ventricular dysfunction questionnaire: Italian translation and validation. Monaldi Arch Chest Dis 2005; 64(2):100-4. doi: 10.4081/monaldi.2005.594 [Crossref] [ Google Scholar]
- Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000; 25(24):3186-91. doi: 10.1097/00007632-200012150-00014 [Crossref] [ Google Scholar]
- Polit DF, Beck CT. The content validity index: are you sure you know what’s being reported? Critique and recommendations. Res Nurs Health 2006; 29(5):489-97. doi: 10.1002/nur.20147 [Crossref] [ Google Scholar]
- Zamanzadeh V, Ghahramanian A, Rassouli M, Abbaszadeh A, Alavi-Majd H, Nikanfar AR. Design and implementation content validity study: development of an instrument for measuring patient-centered communication. J Caring Sci 2015; 4(2):165-78. doi: 10.15171/jcs.2015.017 [Crossref] [ Google Scholar]
- Ayre C, Scally AJ. Critical values for Lawshe’s content validity ratio: revisiting the original methods of calculation. Meas Eval Couns Dev 2014; 47(1):79-86. doi: 10.1177/0748175613513808 [Crossref] [ Google Scholar]
- Gliem JA, Gliem RR. Calculating, interpreting, and reporting Cronbach’s alpha reliability coefficient for Likert-type scales. In: Midwest Research-to-Practice Conference in Adult, Continuing, and Community Education. Columbus, Ohio: Ohio State University; 2003.
- Eghbali-Babadi M, Feizi A, Khosravi A, Nouri F, Taheri M, Sarrafzadegan N. Development and evaluation of the psychometric properties of a hypertension self-care questionnaire. ARYA Atheroscler 2019; 15(5):241-9. doi: 10.22122/arya.v15i5.1835 [Crossref] [ Google Scholar]
- O’Leary CJ, Jones PW. The influence of decisions made by developers on health status questionnaire content. Qual Life Res 1998; 7(6):545-50. doi: 10.1023/a:1008882626075 [Crossref] [ Google Scholar]
- O’Leary CJ. Quantifying the Impact of Left Ventricular Dysfunction on Daily Life and Well Being [dissertation]. St George’s, University of London; 1998.
- Gomes JL, Águeda AF, Heaney A, Duarte C, Lopes C, Costa T. Translation, cross-cultural adaptation and validation of the Osteoarthritis Quality of Life (OAQoL) questionnaire for use in Portugal. Rheumatol Int 2019; 39(4):715-22. doi: 10.1007/s00296-018-4197-8 [Crossref] [ Google Scholar]
- Swaine-Verdier A, Doward LC, Hagell P, Thorsen H, McKenna SP. Adapting quality of life instruments. Value Health 2004; 7 Suppl 1:S27-30. doi: 10.1111/j.1524-4733.2004.7s107.x [Crossref] [ Google Scholar]
- Hagell P, Hedin PJ, Meads DM, Nyberg L, McKenna SP. Effects of method of translation of patient-reported health outcome questionnaires: a randomized study of the translation of the Rheumatoid Arthritis Quality of Life (RAQoL) Instrument for Sweden. Value Health 2010; 13(4):424-30. doi: 10.1111/j.1524-4733.2009.00677.x [Crossref] [ Google Scholar]
- Maneesriwongul W, Dixon JK. Instrument translation process: a methods review. J Adv Nurs 2004; 48(2):175-86. doi: 10.1111/j.1365-2648.2004.03185.x [Crossref] [ Google Scholar]