Arch Iran Med. 26(3):138-146.
doi: 10.34172/aim.2023.22
Original Article
Colorectal Cancer Screening Pilot Project in Tehran-Iran, a Feasibility Study
Hamideh Salimzadeh Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Software, Writing – original draft, 1, 2, * 
Catherine Sauvaget Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing, 3
Alireza Delavari Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Writing – review & editing, 1
Anahita Sadeghi Data curation, Writing – original draft, 1
Mohammad Amani Data curation, Writing – original draft, 1
Sepideh Salimzadeh Data curation, Project administration, Writing – original draft, 1
Azita Karimi Data curation, Project administration, Writing – original draft, 4
Ali Ghanbari Motlagh Supervision, Writing – review & editing, 5
Eric Lucas Project administration, Software, Writing – review & editing, 3
Partha Basu Conceptualization, Writing – review & editing, 3
Reza Malekzadeh Supervision, Writing – review & editing, 1
Author information:
1Digestive Oncology Research Centre, Digestive Disease Research Institute, Tehran University of Medical Sciences, Tehran, Iran
2Department of Surgery, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Sahlgrenska University Hospital, Östra, 416 85, Gothenburg, Sweden
3Early Detection, Prevention & Infections Branch, International Agency for Research on Cancer, 150 Cours Albert Thomas, 69372 Lyon CEDEX 08, France
4Deputy of Health, Tehran University of Medical Sciences, Tehran, Iran
5Cancer Office, Deputy of Health, Ministry of Health, Tehran, Iran
Abstract
Background:
Colorectal cancer (CRC) is the third most common cancer in Iran, where there is no organised CRC-screening programme. This study aimed to evaluate feasibility of CRC screening using a qualitative fecal immunochemical test (FIT) among Iranian average-risk adults.
Methods:
In this feasibility study, 7039 individuals aged 50–75 years were invited by community health workers (CHWs) in southern Tehran and its suburban districts between April 2018 and November 2019. The CHWs performed a qualitative FIT with cut-off level 50 ng Hb/mL buffer and referred those with positive-FIT for colonoscopy to the endoscopy center of Shariati hospital in Tehran. Outcomes included acceptance rate, FIT positivity rate, colonoscopy compliance, detection rates and positive predictive values (PPVs) with 95% confidence interval for CRC and advanced adenomas (AAs).
Results:
Acceptance rate at initial invitation was 71.7%. From 4974 average-risk adults (1600 males and 3374 females) who were offered FIT, 96.8% (n=4813) provided valid samples, of whom 471 (9.8%) tested positive. Among FIT-positive participants, 150 (31.8%) underwent colonoscopy; CRC was detected in 2.0% (n=3) and adenomas in 27.3% (n=41). Detection rate of CRC and AAs per 1000-FIT-screened participants was 0.6 (0.1–1.8) [males: 0.7 (0.01–3.6), females: 0.6 (0.07–2.0)] and 4.2 (2.5–6.4) [males: 5.9 (2.6–11.0), females: 3.4 (1.7–6.0)], respectively. PPVs were 2.0% (0.4–5.7) for CRC and 13.3% (8.3–19.8) for AAs. There was no association between gender and the studied outcomes.
Conclusion:
Our results partially support the feasibility of scaling up organized CRC-screening through the existing healthcare system in Iran; it remains to be discussed carefully to ensure the capacity of healthcare system for adequate colonoscopy services.
Keywords: Colorectal cancer, Feasibility studies, Screening
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Salimzadeh H, Sauvaget C, Delavari A, Sadeghi A, Amani M, Salimzadeh S, et al. Colorectal cancer screening pilot project in Tehran-Iran, a feasibility study. Arch Iran Med. 2023;26(3):138-146. doi: 10.34172/aim.2023.22
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer deaths with over 1.9 million incident cases annually worldwide.1 While the CRC incidence has stabilized over the past few decades among the older population in selected high-income countries thanks to screening,2,3 it has been increasing in low- and middle-income countries.4,5
In Iran, CRC with an age-standardized incidence rate of 15.1 per 100 000 person-years is the third most common cancer6 and an opportunistic screening-based study showed that the prevalence of colonic adenomas in average-risk Iranians was comparable to those in populations that are considered to have a high incidence of CRCs.7 This calls for further investigations for an effective screening strategy and in this regard, stool-base tests such as fecal immunochemical test (FIT) have an acceptable diagnostic yield for CRC in screened populations.8,9 Therefore, we assessed the feasibility of FIT-based screening in average-risk Iranians aged 50–75 years with respect to the existing healthcare structure and challenges regarding implementation of CRC screening within the Iranian healthcare system.
Materials and Methods
Study Setting
This was a feasibility study of the early detection of CRC based on a qualitative FIT. The screening protocol was developed by a joint expert group from the Digestive Diseases Research Institute (DDRI) at Tehran University of Medical Sciences and the International Agency for Research on Cancer (IARC/WHO) in France, considering CRC rates and life expectancy,10 efficacy,11-13 and cost–effectiveness14 of screening in the Iranian context.
A total of 33 primary healthcare centers (PHCs) located in southern Tehran, Shahr-e-Rey, and Eslamshahr were enlisted in the study, involving their associated community health workers (CHWs). We considered approximately 5000 persons in total (~150 per center) to be screened, assuming 70% uptake rate for FIT11 as the main outcome of the study.
The CHWs, who are often from the village or city they serve and entrusted to provide primary healthcare across the country, were in charge of participant recruitment, conducting interviews, risk-assessment, offering and performing FIT, sending reminder calls, arranging referrals, and administering pre-colonoscopy consultation. The CHWs were paid extra for these tasks as they added to their routine workload. Participants aged 50–75 years were invited either in-person from those who visited the PHC for any other reasons or through telephone to those registered in the Iranian health integrated system (Samane Iekparche Behdasht, SIB) held by the Ministry of Health and Medical Education. A written informed consent was obtained from participants after explaining the study objectives and procedures. Average-risk individuals aged 50–75 years who had no documented CRC screening in the last two years were enrolled for FIT screening. We excluded individuals with any of the following criteria from the FIT-screening: inflammatory bowel diseases; a personal history of CRC; family history of CRC in the first-degree relatives; rectal bleeding.8,9 Identification of this subgroup referred to as high-risk individuals in this study was done by the CHW using a risk assessment tool and they were referred directly to the endoscopy center at Shariati Hospital in Tehran.
Screening Procedure
Participants were offered a one-step qualitative FIT (itest, PadyabTeb, Eshtehard Industrial Estate, Iran) with a cut-off value of 50 ng/mL for detection of hemoglobin in stool which required no dietary restrictions.15,16 The CHWs conducted a 25-minute face-to-face interview with each participant to complete the study questionnaire and explain how to obtain stool specimens and return them to the health centers within a maximum of three days after sampling, providing them with an educational pamphlet about stool collection at the end of the interview. One reminder call was sent after one week if the FIT sample was not returned.
In each PHC, FIT testing was performed by the CHWs on the same day of receiving the samples, following the manufacturer’s instructions and the results were obtained within 5 minutes. Samples with invalid results were tested with another test device. The results were immediately notified to the participants on the same day and then a satisfaction questionnaire about the screening program was completed. Individuals with a negative FIT result were recommended to be screened after 2 years. Participants who tested positive were referred to colonoscopy. For this purpose, CHWs conducted a pre-colonoscopy consultation to explain the procedure and bowel preparation, providing them with a prescription for bowel cleansing powders and an instructional pamphlet about colonoscopy, bowel preparation and the contact details of the endoscopy center. Participants were informed that they will receive a telephone call from the endoscopy center within four weeks to set a colonoscopy appointment.
Colonoscopy appointments and patient instructions were conducted by a coordinator in the endoscopy center. If the individuals failed to undergo colonoscopy on the primary scheduled date, the coordinator made a follow-up call to offer a second appointment and ask them about the reasons for not undergoing a colonoscopy. Colonoscopies were performed at Shariati hospital in Tehran by two experienced endoscopists (having performed at least 200 procedures per year) under conscious sedation on pre-scheduled days every week. An anesthesiologist assessed the fitness of the participants for the procedure on the day of colonoscopy. Colonoscopy findings and any complications of the procedure were documented in a standardized report form.
CHWs and the coordinator in the endoscopy center participated in an 8-hour training workshop, reviewing basic information on CRC and screening tests and study protocol in detail. They were encouraged to apply effective health communication to address the participant’s possible concerns regarding screening and to help them proceed with the program.
Financial Implications for the Participants
More than 95% of Iranians have basic health insurance which covers 90% of healthcare expenditures made by public hospitals; however, medical services provided by the private health sector are not fully covered by the basic health insurance, where private insurance companies provide health insurance.17 Information about the screening costs was provided to the participants as part of the informed consent. The costs of FIT test, bowel preparation powders, and transport to the endoscopy center were paid for out of the study budget. The costs of colonoscopy, pathology samples, and cancer treatment were covered by patients depending on their insurance status.
Study Measures and Data Management
Data management and entry were conducted by the research coordinator using an online operating designed software, Research Electronic Data Capture (REDCap) web application18,19 hosted at IARC. Participants’ demographics, knowledge and behavioral parameters, quality of bowel preparation and sedation, colonic lesion features (i.e., number, size, and location), and colonoscopy complications were documented. Advanced adenomas (AAs) were defined as adenomas sized ≥ 10 mm and/or with a villous component, and/or with high grade dysplasia, based on the pathological examinations. Detection rate was defined as the proportion of individuals detected with colonic neoplasms divided by the number of individuals having completed the FIT test, expressed per 1000 FIT-screened persons. The positive predictive value (PPV) of FIT test for AAs or CRC was calculated as the number of participants with AAs or CRC divided by the total number of participants who tested positive for FIT and underwent satisfactory colonoscopy. Incomplete colonoscopy procedures were excluded from the calculations. We surveyed the participants’ satisfaction with the entire FIT screening process, from stool collection at home to submission of samples at the PHCs and receipt of the screening results. The satisfaction questionnaire was developed and validated by specialists in gastroenterology and epidemiology who were involved in the study. Responses were measured using a four-point Likert scale (strongly agree, agree, disagree, strongly disagree). In addition, we investigated the causes of non-compliance with colonoscopy.
Our primary outcomes included acceptance rate, kit return rate, FIT positivity, colonoscopy referral rate, colonoscopy completion, and adenoma, AAs and CRC detection rates, and reasons for non-compliance with colonoscopy. The secondary outcome pertained to the degree of satisfaction of the participants with the screening program.
Statistical Analysis
We applied t-test and χ2 or Fisher’s exact tests for comparing means and proportions, respectively, reporting 95% confidence interval (CI) for estimates. Multivariable logistic regression was used to assess the adjusted effect of factors associated with colonoscopy compliance. Two-tailed tests were applied considering a P value of < 0.05 as statistically significant.
Results
Participants’ Characteristics
The flow diagram of participant recruitment is shown in Figure 1. A total of 7039 individuals aged 50-75 years were invited through PHCs located in southern Tehran, Shahr-e-Rey and Eslamshahr. A total of 5083 (71.7%) invitees accepted to participate in the FIT-screening, out of whom 109 (2.1%) were considered high-risk and referred directly for colonoscopy and the remaining 4,974 average-risk participants received FIT kits between October 2018 and January 2019 (Figure 1).
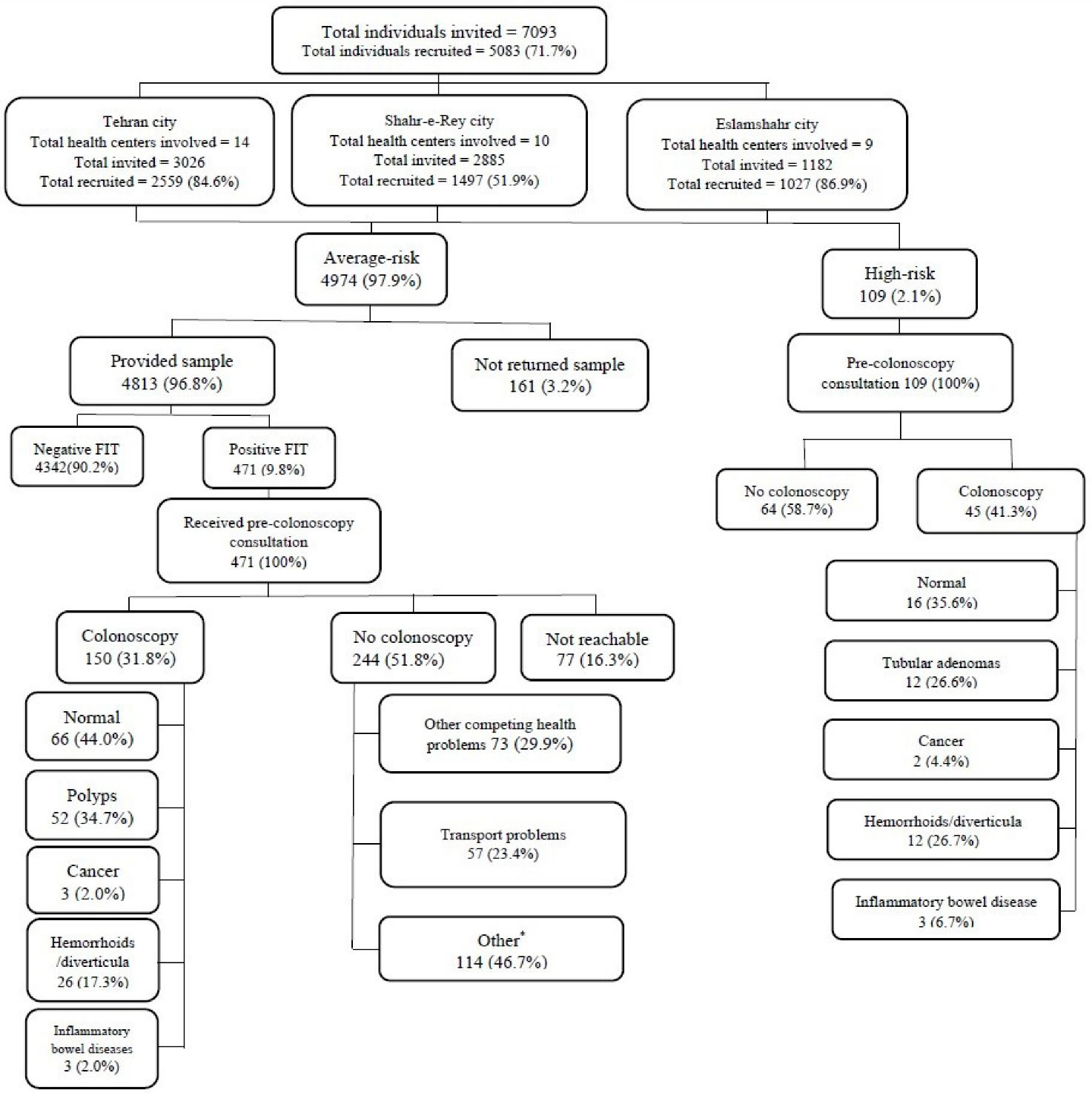
Figure 1.
Recruitment flowchart
*See Table 4
.
Recruitment flowchart
*See Table 4
The mean age of the participants was 59 years (SD = 6.4), and a higher proportion were female (67.8%). Demographic characteristics of the participants by gender are shown in Table 1. Participants were invited mostly when attending PHCs for any reason (72.9%) or through telephone (27.1%). Most of the participants were married (84.5%), homemakers or unemployed (66.7%) and had primary-level or no formal education (71.2%) and basic health insurance (93.6%). The mass media, i.e. radio, television, and newspapers and the internet, were cited by 60.1% as the main source of medical information, 29.8% mostly trusted medical staff, and 10.1% relied on their relatives/friends. Only 1.3% of the participants had been previously screened for CRC (Table 1).
Table 1.
Characteristics of the Participants by Gender
|
|
Male (n=1600)
|
Female (n=3374)
|
Both (n=4974)
|
|
|
No. (%)
|
No. (%)
|
No. (%)
|
| Age (y) |
|
|
|
| 50–54 |
374 (23.3) |
1029 (30.5) |
1403 (28.2) |
| 55–59 |
386 (24.1) |
884 (26.2) |
1270 (25.5) |
| 60–64 |
382 (23.9) |
825 (24.4) |
1207 (24.3) |
| 65–69 |
284 (17.8) |
445 (13.2) |
729 (14.7) |
| 70–75 |
174 (10.9) |
191 (5.7) |
365 (7.3) |
| Registration for screening |
|
|
|
| October-2019 |
109 (0.8) |
205 (6.1) |
314 (6.3) |
| November-2019 |
627 (39.2) |
1359 (40.3) |
1986 (39.9) |
| December-2019 |
579 (36.2) |
1323 (39.2) |
1902 (38.3) |
| January-2020 |
285 (17.8) |
487 (14.4) |
772 (15.5) |
| Invitation method |
|
|
|
| In-person at health centers |
1163 (72.7) |
2462 (72.9) |
3625 (72.9) |
| Phone call |
437 (27.3) |
912 (27.1) |
1372 (27.1) |
| Marital status |
|
|
|
| Married |
1548 (96.8) |
2655 (78.7) |
4203 (84.5) |
| Single/divorced/widow/widower |
52 (3.2) |
719 (21.3) |
771 (15.5) |
| Education |
|
|
|
| No formal education/primary |
921 (57.6) |
2617 (77.5) |
3538 (71.2) |
| Secondary |
564 (35.2) |
691 (20.5) |
1255 (25.2) |
| University |
115 (7.2) |
66 (2.0) |
181 (3.6) |
| Occupation |
|
|
|
| Employed |
73 (4.6) |
42 (1.2) |
115 (2.3) |
| Self-employed |
691 (39.8) |
55 (1.6) |
692 (14.0) |
| Unemployed/homemaker |
133 (8.3) |
3184 (94.4) |
3317 (66.7) |
| Retired |
757 (47.3) |
93 (2.8) |
850 (17.0) |
| Health insurance |
|
|
|
| None |
82 (5.1) |
236 (7.0) |
318 (6.4) |
| Basic |
1153 (72.1) |
2446 (72.5) |
3599 (72.4) |
| Basic plus private |
365 (22.8) |
692 (20.5) |
1057 (21.2) |
| Main source for medical information |
| TV/radio/Internet/newspapers |
967 (60.4) |
2024 (60.0) |
2991 (60.1) |
| Medical staff |
480 (30.0) |
1000 (29.6) |
1480 (29.8) |
| Relatives, friends, other |
153 (9.5) |
350 (10.4) |
503 (10.1) |
| Prior CRC screening |
|
|
|
| Yes |
18 (1.1) |
44 (1.3) |
62 (1.3) |
| No |
1582 (98.9) |
3330 (98.7) |
4912 (98.7) |
Primary Outcomes
A total of 4813 out of 4974 participants who were offered a FIT-kit returned their samples, a total uptake of 96.8%: with 84.6% (n = 4073) returning stool samples within 24 hours of receiving the FIT-kit. Among those who returned samples, only 77 participants (1.6%) had invalid test results for whom samples were re-tested with another device. FIT-testing on the participants showed a positive rate of 9.8% (n = 471). All participants with a positive FIT received pre-colonoscopy consultation and were referred for colonoscopy, of whom 21.9% (103/471) completed their colonoscopy as scheduled. Those who did not come for colonoscopy (n = 368) were contacted and 291 (79.1%) were reachable for a follow-up call and 47 (16.2%) of them underwent a colonoscopy afterwards. The overall compliance rate for colonoscopy was 31.8% (n = 150), with 30.7% (n = 46) having their colonoscopy completed within one month from the referral date. There was no statistically significant association between gender and screening and referral related variables (Table 2).
Table 2.
Screening Process Measures and Intermediate Outcomes
|
|
Male
|
Female
|
Both
|
P
Value
|
|
No.
|
(%)
|
No.
|
(%)
|
No.
|
(%)
|
| Participants registered |
1600 |
|
3374 |
|
4974 |
|
|
| Participants who returned samples |
1537 |
(96.1) |
3276 |
(97.1) |
4813 |
(96.8) |
0.06 |
| Returned within 24 h after receiving kit |
1296 |
(84.3) |
2777 |
(84.8) |
4073 |
(84.6) |
0.3 |
| Returned 24–72 h after receiving kit |
180 |
(11.7) |
348 |
(10.6) |
528 |
(11.0) |
|
| Returned > 72 h after receiving kit |
61 |
(4.0) |
151 |
(4.6) |
212 |
(4.4) |
|
| Invalid FIT test results |
30 |
(2.0) |
47 |
(1.4) |
77 |
(1.6) |
0.4 |
| One repeat test |
28 |
(93.3) |
44 |
(93.6) |
72 |
(93.5) |
|
| Two repeat tests |
2 |
(6.7) |
3 |
(6.4) |
5 |
(6.5) |
1.0 |
| Participants with valid FIT results |
1537 |
|
3276 |
|
4813 |
|
|
| Participants positive on FIT |
152 |
(9.9) |
319 |
(9.7) |
471 |
(9.8) |
0.9 |
| Received pre-colonoscopy consultation |
152 |
(100.0) |
319 |
(100.0) |
471 |
(100.0) |
1.0 |
| Colonoscopy completion after consultation |
30 |
(19.7) |
73 |
(22.9) |
103 |
(21.9) |
0.4 |
| Participants targeted for follow-up calla |
122 |
|
246 |
|
368 |
|
|
| Participants who responded follow-up callb |
96 |
(78.7) |
195 |
(79.3) |
291 |
(79.1) |
0.9 |
| Colonoscopy completion after follow-up call |
16 |
(16.7) |
31 |
(15.9) |
47 |
(16.2) |
0.9 |
| Overall compliance with colonoscopy |
46 |
(30.3) |
104 |
(32.6) |
150 |
(31.8) |
0.6 |
| Poor bowel preparationc |
2 |
(4.3) |
4 |
(3.9) |
6 |
(4.0) |
0.9 |
| Time between referral and colonoscopy completion |
|
|
|
|
|
|
|
| Within 1 month |
12 |
(26.1) |
34 |
(32.7) |
46 |
(30.7) |
0.6 |
| 1-3 months |
23 |
(50.0) |
51 |
(49.0) |
74 |
(49.3) |
|
| > 3 months |
11 |
(23.9) |
19 |
(18.3) |
30 |
(20.0) |
|
FIT, Fecal immunochemical test.
a Participants who did not completed a colonoscopy after pre-colonoscopy consultation.
b n = 77 were not reachable for the following reasons: phone numbers were no longer valid (n = 74), dead (n = 2), and moved (n = 1).
c Among FIT positive participants with normal colonoscopy results.
Overall, 16.8% (n = 835) received a reminder call to return the stool sample and 161 (3.2%) never returned their stool samples for the following reasons: they did not like stool sampling (77.0%), they were unable to collect samples (14.3%), and they did not have time to return samples (8.7%); there was no significant difference (P= 0.6) between genders regarding these reasons (data not shown).
As reported in Table S1 (Supplementary File 1), polyps were detected in 34.7% (52/150), AAs in 13.3% (20/150), and cancer in 2.0% (3/150) of participants undergoing colonoscopy. The detection rates for CRC and AAs per 1000 FIT-screened participants were 0.6 (0.1–1.8) and 4.2 (2.5–6.4), respectively. The PPVs for CRC and AAs were 2.0% (0.4–5.7) and 13.3% (8.3–19.8), respectively (Table S2). The number of polyps per patient varied between 1–3 and 4–7, respectively, for 94.2% and 5.8% of patients detected to have polyps. The polyps measured less than 1 mm for the majority of the patients (67.3%) and ≥ 10 mm for 32.7%. Cecal intubation rate was 99.3% (n = 149/150) and in 4 patients (2.7%) colonoscopy was repeated due to inadequate bowel preparation within 1-month from the first procedure. There was no serious complication related to colonoscopies (data not shown).
None of the demographic and background information were found to be associated with colonoscopy compliance in univariate analysis. We performed multivariable regression analysis including all the variables in the model which indicated no significant differences between participants who completed a colonoscopy and those who did not (Table 3).
Table 3.
Determinants of Compliance to Colonoscopy among the FIT-Positive Participants
|
Characteristics
|
FIT-Positive, n
|
Had Colonoscopy, No. (%)
|
Crude Odds Ratio (95% CI)
|
Adjusted Odds Ratio (95% CI)
|
| Participants registered |
471 |
150 (31.9) |
|
|
| Registration year |
|
|
|
|
| 2018 |
414 |
137 (33.1) |
1.0 (Ref.) |
1.0 (Ref.) |
| 2019 |
57 |
13 (22.8) |
0.6 (0.3–1.1) |
0.6 (0.3–1.1) |
| Age (y) |
|
|
|
|
| 50–59 |
230 |
71 (30.9) |
1.0 (Ref.) |
1.0 (Ref.) |
| 60–69 |
196 |
66 (33.7) |
1.1 (0.7–1.7) |
1.2 (0.7–1.8) |
| 70–75 |
45 |
13 (28.9) |
0.9 (0.6–1.3) |
0.9 (0.6–1.3) |
| Gender |
|
|
|
|
| Female |
319 |
104 (32.6) |
1.0 (Ref.) |
1.0 (Ref.) |
| Male |
152 |
46 (30.3) |
1.1 (0.7–1.6) |
0.9 (0.4–2.1) |
| Marital status |
|
|
|
|
| Single/divorced/widowed |
85 |
26 (30.6) |
1.0 (Ref.) |
1.0 (Ref.) |
| Married |
386 |
124 (32.1) |
1.0 (0.6–1.7) |
1.1 (0.6–1.9) |
| Invitation method |
|
|
|
|
| In-person |
359 |
111 (30.9) |
1.0 (Ref.) |
1.0 (Ref.) |
| Phone call |
112 |
39 (34.8) |
1.1 (0.7–1.8) |
1.1 (0.6–1.7) |
| Occupation |
|
|
|
|
| No job, homemaker |
322 |
105 (32.6) |
1.0 (Ref.) |
1.0 (Ref.) |
| Employed, retired |
149 |
45 (30.2) |
0.8 (0.5–1.3) |
0.8 (0.3–1.7) |
| Education |
|
|
|
|
| None, primary |
366 |
115 (31.4) |
1.0 (Ref.) |
1.0 (Ref.) |
| Secondary and above |
105 |
35 (33.3) |
1.0 (0.9–1.1) |
1.0 (0.9–1.1) |
| Medical insurance |
|
|
|
|
| No |
26 |
7 (26.9) |
1.0 (Ref.) |
1.0 (Ref.) |
| Yes |
445 |
143 (32.1) |
1.2 (0.5–3.1) |
1.2 (0.4–2.9) |
| Kit return time after receiving |
|
|
|
|
| Within 24 h |
382 |
123 (32.2) |
1.0 (Ref.) |
1.0 (Ref.) |
| > 24 h |
89 |
27 (30.3) |
0.9 (0.5–1.5) |
0.9 (0.5–1.5) |
| Source of medical information |
|
|
|
|
| Mass media, Internet |
296 |
96 (32.4) |
1.0 (Ref.) |
1.0 (Ref.) |
| Medical staff, relatives, friends |
175 |
54 (30.9) |
0.9 (0.6–1.3) |
0.9 (0.6–1.3) |
| Prior CRC screening |
|
|
|
|
| Yes |
7 |
2 (28.6) |
1.0 (Ref.) |
1.0 (Ref.) |
| No |
464 |
148 (31.9) |
1.1 (0.2–6.1) |
1.2 (0.2–6.7) |
FIT, Fecal immunochemical test; CI, Confidence interval; Ref, Reference group.
Reasons for non-compliance with colonoscopy among FIT-positive participants are shown in Table 4, which have been classified and presented as logistic barriers (e.g., time limits, scheduling challenges); health system obstacles (e.g., transport problems); and cognitive-emotional barriers (e.g., lack of perceived risk for CRC) (Table 4).
Table 4.
Reasons for Non-compliance to Colonoscopy among FIT Positive Participants
|
|
Male (n=80)
|
Female (n=164)
|
Both (n=244)a
|
|
|
No. (%)
|
No. (%)
|
No. (%)
|
| Logistic barriers |
|
|
|
| Other health problems or more important worries |
28 (35.0) |
45 (27.4) |
73 (29.9) |
| Time limits e.g., cannot have a day off from work |
7 (8.8) |
28 (17.1) |
35 (14.3) |
| Health system barriers |
|
|
|
| Transport problems/no escort to accompany |
9 (11.3) |
48 (29.3) |
57 (23.4) |
| Doctor did not recommend a colonoscopy |
13 (16.2) |
15 (9.1) |
28 (11.5) |
| CHWs are not competent |
3 (3.8) |
5 (3.1) |
8 (3.3) |
| Cognitive-emotional barriers |
|
|
|
| Being young or healthy makes getting cancer less likely |
12 (15.0) |
18 (11.0) |
30 (12.3) |
| Colorectal cancer is incurable, screening is useless |
7 (8.7) |
2 (1.2) |
9 (3.7) |
| Fear of the procedure and detecting cancer |
1 (1.2) |
3 (1.8) |
4 (1.6) |
FIT, fecal immunochemical test; CHWs, Community health workers.
a n = 77 were not reachable for the following reasons: phone number was no longer valid (n = 74), dead (n = 2), and moved (n = 1).
We measured the intervals (days) between: FIT kit collection and sample analysis, referrals and colonoscopy completion, and colonoscopy and final diagnosis according to the registration time, as shown in Table 5. The interval between kit collection and sample analysis showed a relatively stable pattern with time, with a median of one day throughout the study period. The interval between pre-colonoscopy consultation and colonoscopy completion increased with time, with median values ranging between 18.5–55.0 days and concomitantly compliance rate to colonoscopy showed a declining trend. Median values for the time interval between colonoscopy completion and final diagnosis verified by histopathology varied between 10.0–14.0 days (Table 5).
Table 5.
Compliance to Colonoscopy and Intervals Between FIT Delivery, FIT Screening, Colonoscopy, and Final Diagnosis by Registration Period
|
|
Registration Period
|
Participants Assessed, n
|
Mean
|
SD (range)
|
Median (IQR)
|
Colonoscopy done, No. (%)
|
| Interval between FIT kit collection and FIT screening (days) |
October 2018 |
310 |
1.4 |
2.6 (0.0-35.0) |
1.0 (1.0-1.0) |
NA |
| November 2018 |
1926 |
1.4 |
1.6 (0.0-26.0) |
1.0 (1.0-1.0) |
NA |
| December 2018 |
1839 |
1.3 |
1.5 (0.0-32.0) |
1.0 (1.0-1.0) |
NA |
| January 2019 |
738 |
1.3 |
1.5 (0.0-32.0) |
1.0 (1.0-1.0) |
NA |
| Total |
4813 |
1.3 |
1.7 (0.0-35.0) |
1.0 (1.0-1.0) |
NA |
| Interval between pre-colonoscopy consultation and colonoscopy attendance among FIT positive patients (days) |
October 2018 |
8 |
71.6 |
104.7 (5.0-267.0) |
18.5 (7.0-125.0) |
8/31 (25.8) |
| November 2018 |
78 |
64.8 |
80.8 (2.0-394.0) |
39.0 (22.0-61.0) |
78/203 (38.4) |
| December 2018 |
51 |
83.6 |
91.2 (1.0-357.0) |
49.0 (27.0-78.0) |
51/180 (28.3) |
| January 2019 |
13 |
113.4 |
109.4 (12.0-344.0) |
55.0 (35.0-180.0) |
13/57 (22.8) |
| Total |
150 |
75.7 |
88.6 (1.0-394.0) |
42.0 (22.0-66.0) |
150/471 (31.8) |
| Interval between colonoscopy and final diagnosis among patients with pathology samples (days) |
October 2018 |
3 |
15.0 |
8.9 (8.0-25.0) |
12.0 (8.0-25.0) |
NA |
| November 2018 |
34 |
16.4 |
12.1 (5.0-62.0) |
10.5 (8.0-21.0) |
NA |
| December 2018 |
17 |
16.8 |
12.5 (6.0-52.0) |
10.0 (9.0-25.0) |
NA |
| January 2019 |
5 |
13.2 |
5.8 (7.0-21.0) |
14.0 (8.0-16.0) |
NA |
| Total |
59 |
16.2 |
11.5 (5.0-62.0) |
10.0 (8.0-22.0) |
NA |
FIT: fecal immunochemical test; SD: standard deviation; IQR: interquartile range; NA: not applicable.
Secondary Outcomes
Analysis of participants’ opinion and satisfaction level regarding the FIT-screening program indicated that at least 98.6% of the participants were satisfied with the time spent for FIT-testing and the communication and information provided by the CHWs. A remarkable number of participants (99.4%) mentioned that they would recommend FIT to their relatives and friends; 98.7% agreed to repeat FIT after 2 years if tested negative for FIT; 98.2% stated that a free test encouraged them to participate in screening; and 97.9% agreed with the necessity of undergoing a colonoscopy if they tested positive for FIT. CRC was cited as a concerning disease by 95.0% of the participants, 93.5% thought that FIT-testing was easy to do, 91.3% preferred to do the entire FIT-testing (sampling, buffer preparation and FIT reading) at home instead of returning stool samples to the PHCs, and 62.4% mentioned that stool collection was disgusting (Table S3).
Discussion
In the current study, FIT positivity rate was 9.8% with a cut-off value of 50 ng Hb/mL buffer, comparable to our previous study (9.1%) which used a quantitative FIT with a cut-off value of 100 ng Hb/mL.11 In Thailand, a qualitative FIT with a cut-off value of 200 ng Hb/mL led to a positivity rate of 1.1%, far below what was recorded in our study.20 The PPV of FIT for all adenomas per 100 FIT-positive participants was 8.7 in this study which is closely comparable to the respective value of 8.8 in our previous series.11 PPV varies depending on the method and the selected cut-off values of FIT21,22 and higher cut-off values may be even preferable in countries with limited-colonoscopy resources as it offers high PPV for advanced neoplasia and therefore reduces colonoscopy workload.23
The desirable acceptance rate (96.8%) for FIT testing among our participants is comparable to the results (96.0%) of our previous pilot study.11 This could be partly explained by the effective health communication made by CHWs who are believed to build a personalized and trust-based relationship with the participants.24-26 Nevertheless, we recorded a low compliance for colonoscopy which was mainly due to the logistical and health system barriers, comparable to the results from other settings such as Morocco.12,27 In addition, we observed that with time, as the delay between referral to colonoscopy increased, compliance rate with colonoscopy declined. This means that the longer the waiting time for colonoscopy, the lower the compliance rate and the higher the loss-to-follow up. However, these long waiting times seem to be unavoidable, partly due to the increasing demand for colonoscopy associated with a screening program, and partly due to the limited capacity of the endoscopy center which is already limited for diagnostic procedures.
Overall, implementing the first step of CRC screening seems to be feasible in Iran in both organizational and acceptance terms for the following reasons: the favorable test uptake for one-step FIT test, the simplicity of test application, and the well-established healthcare system staffed by trained CHWs.24 However, CRC screening is a multi-step process and Iran will be able to implement screening once the various components of a screening program will be readily available in place, e.g., insured budget at the Ministry of Health, development of screening guidelines and protocols, well-established referral system, improved diagnosis and treatment capacity in terms of staff and facilities, development of a health information system for monitoring, evaluations, and quality assurance. Furthermore, considering the recent challenges imposed by the effects of COVID-19, the low compliance rate for colonoscopy and the low incidence rate of CRC in Iran, we suggest the early diagnosis approach among symptomatic patients and high-risk groups enabling more efficient use of early detection and monitoring resources which eventually reduces unnecessary procedures in low-risk individual.28
Our study has several limitations. First, we could not assess aspects such as cost-effectiveness and equity in the current feasibility study for FIT-screening, which should be thoroughly evaluated before FIT screening is introduced in a subsequent national programme. Second, our study sample was not representative of the general population as females comprised nearly 68.0% of the participants. This is explained by the fact that women visit PHCs more often than men; therefore, a system to invite men other than through PHC visits will be necessary.
In conclusions,based on our results, FIT modality as a test of choice for CRC screening can be a safe and acceptable method of screening among the Iranian average-risk population. However, the suboptimal compliance rate with colonoscopy outweighs the advantages of FIT screening. We therefore suggest improving awareness in the general population with an early diagnosis approach among symptomatic patients and high-risk individuals. The results of the current study may not be limited to Iranians and could have implications for other developing countries with similar trends in the CRC epidemic.
Supplementary File
Supplementary file 1 contains Tables S1-S3.
(pdf)
Acknowledgements
The authors are grateful for the support from the CHWs at the selected health centers. We also thank Ms. Krittika Guinot at the International Agency for Research on Cancer for her help in drafting the study protocol.
Competing Interests
The authors declare that they have no conflict of interest.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Ethical Approval
The study was approved by the National Institute for Medical Research Development (Ref.: IR.NIMAD.REC.1396.158, Protocol Identification: 962466, EC) and the IARC Ethics Committee (Ref.: IEC Meeting 2018-03, IEC Project No. 18-17). Data collected in this study were processed anonymously.
Funding
This work was supported by the National Institute for Medical Research Development (NIMAD) [grant number 962466].
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3):209-49. doi: 10.3322/caac.21660 [Crossref] [ Google Scholar]
- Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019; 68(12):2179-85. doi: 10.1136/gutjnl-2019-319511 [Crossref] [ Google Scholar]
- Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019; 68(10):1820-6. doi: 10.1136/gutjnl-2018-317592 [Crossref] [ Google Scholar]
- Safiri S, Ghajarieh Sepanlou S, Ikuta KS, Bisignano C, Salimzadeh H, Delavari A. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2019; 4(12):913-33. doi: 10.1016/s2468-1253(19)30345-0 [Crossref] [ Google Scholar]
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66(4):683-91. doi: 10.1136/gutjnl-2015-310912 [Crossref] [ Google Scholar]
- Roshandel G, Ferlay J, Ghanbari-Motlagh A, Partovipour E, Salavati F, Aryan K. Cancer in Iran 2008 to 2025: recent incidence trends and short-term predictions of the future burden. Int J Cancer 2021; 149(3):594-605. doi: 10.1002/ijc.33574 [Crossref] [ Google Scholar]
- Delavari A, Bishehsari F, Salimzadeh H, Khosravi P, Delavari F, Nasseri-Moghaddam S. Adenoma detection rates in an opportunistic screening colonoscopy program in Iran, a country with rising colorectal cancer incidence. BMC Gastroenterol 2014; 14:196. doi: 10.1186/s12876-014-0196-8 [Crossref] [ Google Scholar]
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021; 325(19):1978-98. doi: 10.1001/jama.2021.4417 [Crossref] [ Google Scholar]
- Young GP, Symonds EL, Allison JE, Cole SR, Fraser CG, Halloran SP. Advances in fecal occult blood tests: the FIT revolution. Dig Dis Sci 2015; 60(3):609-22. doi: 10.1007/s10620-014-3445-3 [Crossref] [ Google Scholar]
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2020.
- Salimzadeh H, Bishehsari F, Sauvaget C, Amani M, Hamzehloo G, Nikfarjam A. Feasibility of colon cancer screening by fecal immunochemical test in Iran. Arch Iran Med 2017; 20(12):726-33. [ Google Scholar]
- Salimzadeh H, Delavari A, Montazeri A, Mirzazadeh A. Knowledge and practice of Iranians toward colorectal cancer, and barriers to screening. Int J Prev Med 2012; 3(1):29-35. [ Google Scholar]
- Salimzadeh H, Eftekhar H, Majdzadeh R, Montazeri A, Shojaeizadeh D, Delavari A. More than half of senior residents in Tehran have never heard about colorectal cancer screening. Asian Pac J Cancer Prev 2011; 12(11):2851-6. [ Google Scholar]
- Javadinasab H, Daroudi R, Salimzadeh H, Delavari A, Vezvaie P, Malekzadeh R. Cost-effectiveness of screening colonoscopy in Iranian high risk population. Arch Iran Med 2017; 20(9):564-71. [ Google Scholar]
- Hoepffner N, Shastri YM, Hanisch E, Rösch W, Mössner J, Caspary WF. Comparative evaluation of a new bedside faecal occult blood test in a prospective multicentre study. Aliment Pharmacol Ther 2006; 23(1):145-54. doi: 10.1111/j.1365-2036.2006.02702.x [Crossref] [ Google Scholar]
- Trojan J, Povse N, Schröder O, Stein J. A new immunological test strip device for the rapid, qualitative detection of faecal occult blood. Z Gastroenterol 2002; 40(11):921-4. doi: 10.1055/s-2002-35415 [Crossref] [ Google Scholar]
- Doshmangir L, Bazyar M, Rashidian A, Gordeev VS. Iran health insurance system in transition: equity concerns and steps to achieve universal health coverage. Int J Equity Health 2021; 20(1):37. doi: 10.1186/s12939-020-01372-4 [Crossref] [ Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42(2):377-81. doi: 10.1016/j.jbi.2008.08.010 [Crossref] [ Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. doi: 10.1016/j.jbi.2019.103208 [Crossref] [ Google Scholar]
- Khuhaprema T, Sangrajrang S, Lalitwongsa S, Chokvanitphong V, Raunroadroong T, Ratanachu-Ek T. Organised colorectal cancer screening in Lampang province, Thailand: preliminary results from a pilot implementation programme. BMJ Open 2014; 4(1):e003671. doi: 10.1136/bmjopen-2013-003671 [Crossref] [ Google Scholar]
- Sarkeala T, Färkkilä M, Anttila A, Hyöty M, Kairaluoma M, Rautio T. Piloting gender-oriented colorectal cancer screening with a faecal immunochemical test: population-based registry study from Finland. BMJ Open 2021; 11(2):e046667. doi: 10.1136/bmjopen-2020-046667 [Crossref] [ Google Scholar]
- Ponti A, Anttila A, Ronco G, Senore C. Cancer Screening in the European Union. Report on the Implementation of the Council Recommendation on Cancer Screening. 2017. Available from: https://health.ec.europa.eu/document/download/911ecf9b-0ae2-4879-93e6-b750420e9dc0_en.
- Fraser CG, Halloran SP, Allison JE, Young GP. Making colorectal cancer screening FITTER for purpose with quantitative faecal immunochemical tests for haemoglobin (FIT). Clin Chem Lab Med 2013; 51(11):2065-7. doi: 10.1515/cclm-2013-0408 [Crossref] [ Google Scholar]
- Sadegh Tabrizi J, Pourasghar F, Gholamzadeh Nikjoo R. Status of Iran’s primary health care system in terms of health systems control knobs: a review article. Iran J Public Health 2017; 46(9):1156-66. [ Google Scholar]
- Christy SM, Davis SN, Williams KR, Zhao X, Govindaraju SK, Quinn GP. A community-based trial of educational interventions with fecal immunochemical tests for colorectal cancer screening uptake among blacks in community settings. Cancer 2016; 122(21):3288-96. doi: 10.1002/cncr.30207 [Crossref] [ Google Scholar]
- Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health 2005; 82(2):216-24. doi: 10.1093/jurban/jti046 [Crossref] [ Google Scholar]
- Selmouni F, Amrani L, Sauvaget C, Bakkar M, El Khannoussi B, Souadka A. Delivering colorectal cancer screening integrated with primary health care services in Morocco: lessons learned from a demonstration project. Cancer 2022; 128(6):1219-29. doi: 10.1002/cncr.34061 [Crossref] [ Google Scholar]
- Hull MA, Rees CJ, Sharp L, Koo S. A risk-stratified approach to colorectal cancer prevention and diagnosis. Nat Rev Gastroenterol Hepatol 2020; 17(12):773-80. doi: 10.1038/s41575-020-00368-3 [Crossref] [ Google Scholar]