Arch Iran Med. 25(9):617-623.
doi: 10.34172/aim.2022.97
Original Article
Clinical Evaluation of an HTK Solution for Liver Transplantation: A Phase 3 Randomized Pilot Clinical Trial Study
Seyed Ali Malekhosseini Resources, Writing – review & editing, 1 
Younes Ghasemi Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing, 2, 3, * 
Javad Rousta Investigation, Project administration, Writing – review & editing, 2
Roghayyeh Aghaei Investigation, Writing – review & editing, 2
Sedigheh Kianpour Investigation, Writing – review & editing, 2
Manica Negahdaripour Conceptualization, Investigation, Resources, Writing – review & editing, 2, 3 
Reza Heidari Investigation, Writing – review & editing, 2
Alireza Shamsaeefar Investigation, Writing – review & editing, 1
Siavash Gholami Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing, 1
Saman Nikeghbalian Investigation, Methodology, Writing – review & editing, 1, * 
Author information:
1Shiraz Transplant Center, Abu Ali Sina Hospital, Shiraz University of Medical Sciences, Shiraz, Iran
2Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
3Department of Pharmaceutical Biotechnology, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
Abstract
Background:
Organ preservation solutions are not easily accessible in Iran, similar to many resource-limited countries. We aimed to evaluate the efficacy of a locally-produced HTK solution among adult liver transplantation candidates in a pilot clinical trial study.
Methods:
Adult patients undergoing liver transplantation were randomly allocated into two groups. One received the HTK solution (PharMedCina Inc., Shiraz, Iran), and the second received the commercially available HTK solution (Custodiol®).
Results:
Overall, 28 individuals entered the study, including 11 and 9 males (78.6% and 64.3%) in the Custodiol® and local HTK groups, respectively. Clinical characteristics, including postoperative biliary complications, reperfusion syndrome, infection and primary non-function (PNF) rates, amount of intraoperative bleeding, length of hospital and ICU stay, peak aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and duration of follow-up were similar between the two groups (P>0.05). One patient died in the locally-produced HTK group. The patient underwent re-transplantation 20 days after his first liver transplantation due to PNF. Two patients died in the Custodiol group, both due to PNF of the liver, which occurred five and three days after transplantation. The two groups did not show any difference regarding serum levels of AST, ALT, alkaline phosphatase (ALP), bilirubin, platelet count, prothrombin time and international normalized ratio, white blood cell count, blood urea nitrogen, and creatinine on the first postoperative day and on the day of discharge (P>0.05).
Conclusion:
Based on the findings of this pilot study with the current sample size, no statistically significant difference was found between our locally-produced HTK solution and Custodiol® regarding clinical outcomes
Keywords: Clinical trial, HTK, Organ transplantation, Preservation solution, Prognosis, Transplantation
Copyright and License Information
© 2022 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Malekhosseini SA, Ghasemi Y, Rousta J, Aghaei R, Kianpour S, Negahdaripour M, et al. Clinical evaluation of an htk solution for liver transplantation: A phase 3 randomized pilot clinical trial study. Arch Iran Med. 2022;25(9):617-623. doi: 10.34172/aim.2022.97
Introduction
According to the World Health Organization (WHO), more than 140 000 organ transplantations are performed each year globally.1 Organ transplantation is a complex and delicate practice performed in specific medical centers in the world. A successful organ transplantation depends on multiple factors, among which quality of the organ is one of the leading factors that determine the success of transplantation.2
Organ preservation solutions are among the most important factors that insure the function of an organ after procurement. The solution plays a vital role in ischemia-reperfusion injury and significantly affects primary function of the transplanted organ.3,4 Different preservation solutions have been introduced throughout decades, which have been used intermittently in clinical settings. The first was the University of Wisconsin or UW solution, which was first introduced in 1987,5 after which the Histidine-Tryptophan-Ketoglutarate (HTK) solution was presented as a potential replacement in 1990. Today, some commonly used and commercially available preservation products include Euro-Collins, UW (Viaspan®), Celsior®, Custodiol®, and IGL-1®.2
The HTK solution has shown some advantages over the UW solution, including lower costs, as well as lower potassium and viscosity, which has made it a more suitable replacement in clinical settings.6 Furthermore, studies have shown a lower incidence of biliary complications following HTK usage compared to the UW preservation solution.7
In Iran, despite advancements in surgery and the availability of a national program for transplantation, similar to many resource-limited countries, access to preservation solutions is difficult. Thus, we formulated a local preservation solution, similar in biological structure to the HTK solution.
In this study, we aimed to evaluate the efficacy of the locally produced HTK solution among a population of adult liver transplantation candidates in a pilot phase 3 clinical trial study.
Materials and Methods
Study Setting
This study was conducted in the Shiraz Transplant Center affiliated to Shiraz University of Medical Sciences, Shiraz, Iran. Patients undergoing liver transplantation who were above 18 years of age and gave their informed consent to enter the study, were considered for inclusion in this trial.
Patients with organ donations from outside of the province (in order to limit the cold ischemic time of organs), pregnant or lactating patients, patients with donations after cardiac death, those who were undergoing re-transplantations, and those with machine perfusion, were excluded from the study.
After entry into the study, patients were randomly allocated in one of the two following groups. The HTK solution (PharMedCina Inc., Shiraz, Iran) was used for organ preservation before transplantation in one group, and the commercially available HTK solution (Custodiol®) was utilized in the second group.
Randomization
The eligible study participants (n = 28) were randomized into two study arms (1:1) using the permuted block randomization with a fixed block size of 2. The randomization sequence was generated with an online program available at https://www.sealedenvelope.com/simple-randomiser/v1/lists.
Allocation Concealment
The generated random sequences were inserted in opaque envelops enumerated in sequence from 01 to 28, each of which was used for consecutive study participants.
Blinding and Sample Size
-
Patients were blinded to the preservation solution used in their liver transplantation.
-
In this pilot study, a sample size of 14 individuals was considered for each treatment arm.
Primary Outcomes
Secondary Outcomes
-
Changes in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) from the first day after transplantation up to the day of discharge from hospital
-
Acute rejection during first month after transplantation
-
Early patient survival (three months)
-
Biliary complications
Follow-up
Patients were followed according to their routine transplantation follow-up schedule. This includes a daily visit for the first week, which changes to visits on every other day during the second post-transplantation week. Doppler sonography is done for patients during the second week. After one month of hospitalization, the patients are sent home and during the following two months, they are visited every two weeks, which then becomes every six months. In case of any complaints or complications, patients are asked to refer to a medical health center and transplantation coordinators are readily available at all times to answer patients’ questions or to schedule a visit in the center for patients.
Considering the short-term primary and secondary outcomes, the minimum follow-up for each patient included in this study was considered three months.
Variables
Patients’ data were gathered using a predefined data gathering sheet, which included data on baseline and clinical characteristics. Baseline characteristics including age, sex, body mass index (BMI), and blood type, clinical characteristics including underlying disease, duration of liver disease prior to transplantation, cause of liver failure, and Model for End-Stage Liver Disease (MELD) score, surgery related data including type of organ transplantation (deceased or living organ donation), type of hepatectomy used, and warm and cold ischemic time, clinical outcomes including rate of reperfusion syndrome, postoperative complications, in-hospital rejection, PNF, amount of bleeding during surgery, length of hospital and ICU stay, and mortality were documented for each individual. Furthermore, data on the patients’ laboratory tests were registered on consecutive days from the day of transplantation to discharge in order to document changes. Donor related data including age and sex were also gathered for each patient.
Statistical Analysis
Data was analyzed using the Statistical Software for Social Sciences (SPSS Inc., Chicago, IL, USA), for Windows, version 20. The intention-to-treat protocol was used for data analysis. Qualitative data were compared between the two groups using the χ2 test or the Fisher’s exact test, when necessary. Quantitative data with a normal distribution were compared between the groups using the independent t test and variables without a normal distribution were compared using the Mann-Whitney U test. For comparison of survival between the two groups, the Kaplan-Meier plot and the log-rank test were used.
Results
In total, 28 individuals entered the study, including 11 males (78.6%) in the Custodiol HTK group and 9 males (64.3%) in the local HTK group. Mean (SD) age in the local and Custodiol HTK groups was 46.2 ± 13.9 years and 48.0 ± 8.1 years, respectively. Overall, the most common causes of liver failure in the study groups were non-alcoholic steatohepatitis (NASH) (7/28), PSC (4/28), autoimmune hepatitis (3/28), and hepatitis B virus (HBV) (3/28). The two groups did not show any difference in baseline clinical characteristics. The baseline and clinical characteristics of patients are shown in Tables 1 and 2.
Table 1.
Baseline Characteristics of the Study Population
|
Characteristics
|
CustodiolHTK (n=14)
|
Local HTK (n=14)
|
Mean Difference (95% CI)
|
Effect Size (95% CI)
|
P
Value
|
| Age, years |
48.0 ± 8.1 |
46.2 ± 13.9 |
1.7 (-7.1–10.5) |
0.15 (-0.59–0.89) |
0.694 |
| Sex, No. (%) |
| Male |
11 (78.6) |
9 (64.3) |
— |
— |
0.678 |
| Female |
3 (21.4) |
5 (35.7) |
— |
— |
| BMI, kg/m2 |
24.9 ± 2.3 |
24.9 ± 3.3 |
-0.007 (-2.2–2.2) |
-0.002 (-0.74–0.73) |
0.995 |
| Blood type, No. (%) |
| O |
8 (57.1) |
8 (57.1) |
— |
— |
0.900 |
| A |
3 (21.4) |
3 (21.4) |
— |
— |
| B |
2 (14.3) |
2 (14.3) |
— |
— |
| AB |
1 (7.1) |
1 (7.1) |
— |
— |
| Underlying disease, No. (%) |
| Cardiac |
1 (7.1) |
0 |
— |
— |
0.633 |
| Diabetes |
2 (14.3) |
4 (28.6) |
— |
— |
| Renal failure |
1 (7.1) |
1 (7.1) |
— |
— |
| Duration of liver disease, months |
55.1 ± 46.5 |
54.4 ± 64.2 |
0.6 (-51.1–52.3) |
0.01 (-0.84–0.86) |
0.979 |
| Cause of liver failure, No. (%) |
| Nonalcoholic steatohepatitis |
3 (21.4) |
4 (28.6) |
— |
— |
0.464 |
| Primary sclerosing cholangitis |
4 (28.5) |
0 |
— |
— |
| Cryptogenic |
0 |
2 (14.3) |
— |
— |
| Autoimmune hepatitis |
2 (14.3) |
1 (7.1) |
— |
— |
| Hepatitis C virus |
1 (7.1) |
0 |
— |
— |
| Alcoholic hepatitis |
0 |
1 (7.1) |
— |
— |
| Budd Chiari |
0 |
1 (7.1) |
— |
— |
| Hepatitis B virus |
2 (14.3) |
1 (7.1) |
— |
— |
| Hepatitis B virus + Hepatocellular carcinoma |
1 (7.1) |
0 |
— |
— |
| Hyperoxalemia |
0 |
1 (7.1) |
— |
— |
| Primary biliary cirrhosis |
1 (7.1) |
1 (7.1) |
— |
— |
| Primary sclerosing cholangitis + Hepatocellular carcinoma |
0 |
1 (7.1) |
— |
— |
| Wilson's disease |
0 |
1 (7.1) |
— |
— |
| MELD score |
18.3 ± 5.1 |
17.1 ± 3.9 |
1.1 (-2.5–4.9) |
0.25 (-0.53–1.04) |
0.525 |
| Type of organ transplantation, No. (%) |
| Deceased |
13 (92.9) |
10 (71.4) |
— |
— |
0.326 |
| Living |
1 (7.1) |
4 (28.6) |
— |
— |
| Type of hepatectomy, No. (%) |
| Piggyback |
11 (78.6) |
12 (85.7) |
— |
— |
0.622 |
| Standard |
3 (21.4) |
2 (14.3) |
— |
— |
| Warm ischemic time; minutes |
36.2 ± 10.2 |
36.9 ± 5.2 |
-4.4 (-14.4–5.4) |
-0.08 (-0.82–0.65) |
0.818 |
| Cold ischemic time; minutes |
243.5 ± 190.3 |
197.5 ± 183.3 |
214.2 (-143.3–51.9) |
0.24 (-0.50–0.98) |
0.520 |
BMI, body mass index; MELD, model for end-stage liver disease.
All plus-minus are means and standard deviation unless stated otherwise.
Table 2.
Clinical Characteristics and Outcomes of Patients
|
Characteristics
|
CustodiolHTK (n=14)
|
Local HTK (n=14)
|
Mean Difference (95% CI)
|
Effect Size (95% CI)
|
P
Value
|
| Reperfusion syndrome, No. (%) |
| Yes |
7 (50) |
5 (41.7) |
— |
— |
0.671 |
| No |
7 (50) |
7 (58.3) |
— |
— |
| Postoperative complications, No. (%) |
| Bleeding |
0 |
2 (14.3) |
— |
— |
0.219 |
| Bleeding + portal thrombosis |
1 (7.1) |
0 |
— |
— |
| Infections, No. (%) |
| None |
8 (57.1) |
11 (78.6) |
— |
— |
0.483 |
| Site of surgery |
0 |
1 (7.1) |
— |
— |
| Cytomegalovirus |
1 (7.1) |
0 |
— |
— |
| Pneumonia |
1 (7.1) |
1 (7.1) |
— |
— |
| Urinary |
3 (21.4) |
1 (7.1) |
— |
— |
| Sepsis |
1 (.1) |
0 |
— |
— |
| In-hospital rejection, No. (%) |
| Yes |
2 (14.3) |
5 (35.7) |
— |
— |
0.385 |
| No |
12 (85.7) |
9 (64.3) |
— |
— |
| Primary non-function, No. (%) |
| Yes |
2 (14.3) |
1 (7.1) |
— |
— |
0.541 |
| No |
12 (85.) |
13 (92.9) |
— |
— |
| Biliary complications during hospitalization; n (%) |
| Biloma |
0 |
1 (7.1) |
— |
— |
0.365 |
| Stricture |
1 (7.1) |
0 |
— |
— |
| No |
13 (92.9) |
13 (92.9) |
— |
— |
| Amount of bleeding, cc |
2084 ± 987 |
1700 ± 1813 |
384 (-809–1579) |
0.26 (-0.52–1.05) |
0.512 |
| ICU stay, days |
10 ± 4.2 |
7.9 ± 2.7 |
2.0 (-0.7–4.9) |
0.58 (-0.19–1.34) |
0.145 |
| Hospital stay, days |
12.1 ± 5.5 |
14.0 ± 3.2 |
-1.8 (-5.4–1.7) |
-0.40 (-1.16–0.36) |
0.303 |
| Peak aspartate aminotransferase, IU/L |
1214 ± 694 |
1101 ± 1175 |
113 (-859–1085) |
0.11 (-0.74–1.01) |
0.799 |
| Peak alanine transaminase, IU/L |
899 ± 499 |
682 ± 599 |
21 (-314–49) |
0.39 (-0.52–1.30) |
0.401 |
| Duration of follow-up, days |
344 ± 166 |
265 ± 142 |
79 (-40–200) |
-0.61 (-1.37–0.14) |
0.185 |
| Mortality, No. (%) |
| Yes |
2 (14.3) |
1 (7) |
— |
— |
0.541 |
| No |
12 (85.7) |
13 (93) |
— |
— |
ICU, Intensive care unit.
All plus-minus are means and standard deviation unless stated otherwise.
The two groups were similar regarding clinical characteristics including postoperative biliary complications, reperfusion syndrome, infection rates, PNF rates, amount of intraoperative bleeding, length of hospital and ICU stay, peak AST and ALT, and duration of follow-up (P > 0.05).
One patient died in the locally produced HTK group. The patient had a re-transplantation 20 days after his first liver transplantation due to PNF, and the patient died three days after his re-transplantation due to another PNF of the liver. For the re-transplantation, the patient received the Custodiol HTK solution. Two patients died in the Custodiol HTK group, both due to PNF of the liver, which occurred five and three days after transplantation. One of the patients underwent re-transplantation on the fifth day of his primary transplantation and died on the same day (survival rate 92.9% for the local HTK group vs. 85.7% for the Custodiol HTK group; P = 0.520) (Figure 1).
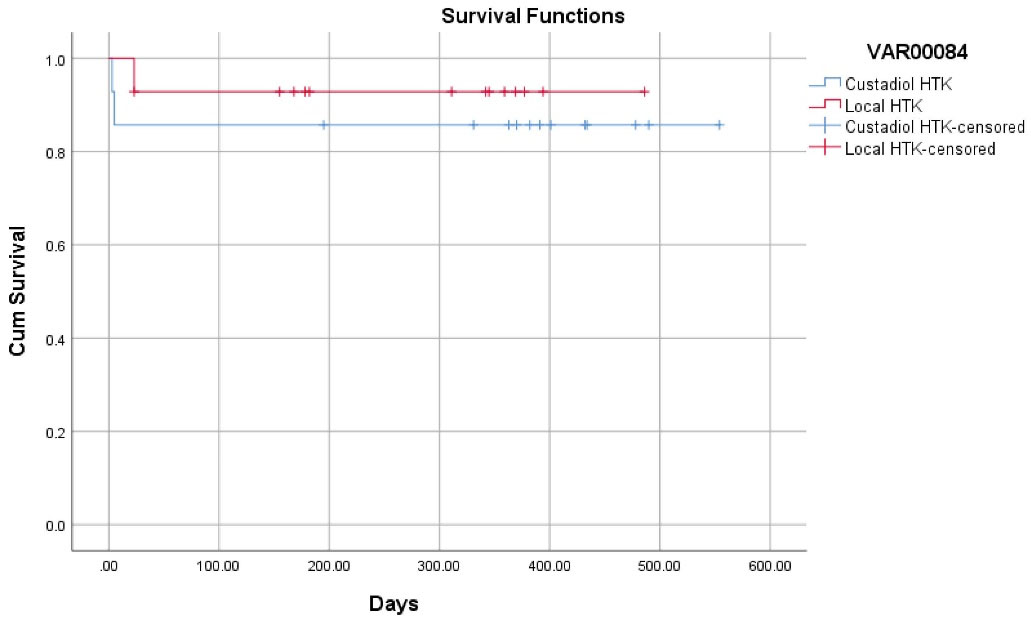
Figure 1.
Kaplan Kaplan-Meier Plot for Survival in the Two Comparison Groups
.
Kaplan Kaplan-Meier Plot for Survival in the Two Comparison Groups
Regarding laboratory tests on consecutive days after receiving the HTK solutions, the two groups did not show any difference in serum levels of AST, ALT, alkaline phosphatase (ALP), bilirubin, platelet count, prothrombin time and international normalized ratio, white blood cell count, blood urea nitrogen, and creatinine on the first postoperative day and on the day of discharge (P > 0.05) (Table 3).
Table 3.
Laboratory Tests on Postoperative Days
|
Variables
|
CustodiolHTK (n=14)
|
Local HTK (n=14)
|
Mean Difference (95% CI)
|
Effect Size (95% CI)
|
P
Value
|
| Total bilirubin - mg/dL |
Day 1 |
6.8 ± 5.0 |
4.4 ± 6.5 |
2.4 (-2.0–7.0) |
0.42 (-0.33–1.16) |
0.273 |
| Day 7 |
3.1 ± 2.7 |
1.0 ± 0.5 |
2.0 (0.49–3.6) |
1.09 (0.23–1.92) |
0.012 |
| On discharge |
2.0 ± 1.4 |
1.6 ± 3.3 |
0.4 (-1.7–2.5) |
0.16 (-0.61–0.93) |
0.682 |
| AST (IU/L) |
Day 1 |
1506 ± 1539 |
999 ± 1019 |
507 (-506–1521) |
0.38 (-0.36–1.13) |
0.313 |
| Day 7 |
71.4 ± 74.1 |
65.1 ± 25.1 |
6.3 (-39.0–51.6) |
0.11 (-0.68–0.92) |
0.776 |
| On discharge |
41.9 ± 30.9 |
32.3 ± 16.3 |
9.5 (-10.9–30.0) |
0.39 (-0.42–1.20) |
0.345 |
| ALT (IU/L) |
Day 1 |
929 ± 644 |
630 ± 519 |
298 (-163–760) |
0.51 (-0.26–1.2) |
0.196 |
| Day 7 |
228 ± 188 |
180 ± 115 |
47 (-81–177) |
0.31 (-0.49–1.11) |
0.454 |
| On discharge |
121 ± 5 |
113 ± 66 |
8 (-50–66) |
0.11 (-0.67–0.90) |
0.771 |
| ALP (IU/L) |
Day 1 |
414 ± 487 |
365 ± 321 |
49 (-275–374) |
0.12 (-0.63–0.87) |
0.757 |
| Day 7 |
448 ± 295 |
423 ± 258 |
25 (-208–259) |
0.09 (-0.71–0.89) |
0.824 |
| On discharge |
470 ± 348 |
440 ± 389 |
-29 (-280–340) |
0.08 (-0.71–0.90) |
0.844 |
| PT (s) |
Day 1 |
29.3 ± 13.2 |
24.1 ± 9.2 |
5.2 (-3.7–14.1) |
0.46 (-0.30–1.22) |
0.242 |
| Day 7 |
15.0 ± 2.4 |
13.7 ± 1.5 |
1.2 (-1.2–3.7) |
0.58 (-0.50–1.65) |
0.299 |
| On discharge |
14.7 ± 1.9 |
15.8 ± 5.8 |
-1.1 (-8.3–6.0) |
-0.26 (-1.45–0.93) |
0.669 |
| INR |
Day 1 |
2.6 ± 1.1 |
1.9 ± 0.4 |
0.6 (-0.006-1.3) |
0.78 (-0.006–1.56) |
0.052 |
| Day 7 |
1.2 ± 0.2 |
1.1 ± 0.1 |
0.1 (-0.08–0.3) |
-0.72 (-0.38–1.80) |
0.206 |
| On discharge |
1.1 ± 0.1 |
1.2 ± 0.4 |
-0.09 (-0.5–0.3) |
-0.28 (-1.46–0.92) |
0.654 |
| White blood cell count |
Day 1 |
12230 ± 4993 |
12007 ± 4451 |
-776 (-4520–2967) |
-0.16 (-0.91–0.59) |
0.673 |
| Day 7 |
8845 ± 4093 |
8108 ± 4590 |
737 (-2976–4451) |
0.16 (-0.63–0.9) |
0.685 |
| On discharge |
8263 ± 2971 |
8314 ± 4448 |
-51 (-3281–3179) |
-0.01 (-0.80–0.77) |
0.974 |
| Platelet count (per microliter) |
Day 1 |
96384 ± 113672 |
77214 ± 59732 |
19.170 (-55115–93455) |
0.21 (-0.54–0.96) |
0.584 |
| Day 7 |
93000 ± 69951 |
97538 ± 63481 |
-4538 (-6159–52682) |
-0.06 (-0.87–0.73) |
0.869 |
| On discharge |
138363 ± 62944 |
181500 ± 121521 |
-43136 (-132444–4611) |
-0.37 (-1.16–0.42) |
0.361 |
| BUN level (mg/dL) |
Day 1 |
20.2 ± 9.7 |
28 ± 13.9 |
-7.7 (-17.2–1.7) |
-0.64 (-1.41–0.13) |
0.108 |
| Creatinine level (mg/dL) |
Day 1 |
1.1 ± 0.6 |
1.5 ± 1.0 |
-0.3 (-1.0–0.3) |
0.17 (-0.66–1.01) |
0.278 |
AST, Aspartate aminotransferase; ALP, alkaline phosphatase; PT, prothrombin time; INR, International normalized ratio; BUN, blood urea nitrogen.
All plus-minus are means and standard deviation unless stated otherwise.
The mean donor age in the local and Custodiol HTK groups was 41.9 ± 18.3 years and 44.8 ± 18.7 years (mean difference: 2.9, 95% CI = -11.1–17.0), respectively. Donor specifics are presented in Table 4.
Table 4.
Donor Related Characteristics Among the Study Population
|
Variables
|
Custodiol HTK (n=14)
|
Local HTK (n=14)
|
Mean Difference (95% CI)
|
P
Value
|
| Age (years) |
44.8 ± 18.7 |
41.9 ± 18.3 |
2.9 (-11.1–17.0) |
0.673 |
|
Sex; n (%)
|
| Male |
10 (69.2) |
13 (92.9) |
- |
0.326 |
| Female |
4 (23.1) |
1 (7.1) |
- |
Discussion
In this study, a generic locally produced HTK solution was evaluated and its clinical efficacy was compared to that of a commercially available and well-established standard Custodiol HTK solution in a clinical trial. Accordingly, the two solutions were found to be similar regarding clinical outcomes among adult patients undergoing liver transplantation.
In literature, HTK solutions have been mostly compared to UW preservation products with regard to clinical outcomes after transplantation in the settings of clinical trial studies,8,9 and some have focused on developing new formulations and preservation solutions.10-12
The effect of preservation solutions on the organ is mainly considered to be short-term. One of the most important aspects to consider with preservations solutions in clinical settings is the post-transplantation biliary complications. One mechanism through which preservation solutions are believed to contribute to the occurrence of biliary complications is the role of the solution in better preservation of the bile ducts, which consequently leads to biliary complications. One report concluded that the preservation solution used for organ preservation even plays a more important role than the cold ischemic time in prevention of postoperative biliary stricture.13
The rate of PNF after using HTK solutions in liver transplantation has been variable in different clinical trial studies depending on the study sample and design14-20 and graft dysfunction has been reported to be up to 27% in literature.21 More specifically, occurrence of organ dysfunction is believed to be dependent on multiple factors including ischemia time, quality of the organ (lower with marginal organs), immunological matching, and graft reperfusion, which includes the type of preservation solution.21 Literature has shown that occurrence of PNF is considered to be unrelated to any factors related to surgery and is highly dependent on the organ preservation method used.22 In our study, two patients (14.2%) in the local HTK group and one patient (7.1%) in our Custodiol HTK group developed PNF. Occurrence of PNF is a relatively rare entity itself, and according to an unpublished report, the rate of PNF among adult liver transplantation recipients in our center is between 5 to 11%.
Different studies have reported variable one-year survival rates among patient with the use of the HTK preservation products. The overall one-year survival rate in our center among adult liver transplantation recipients is reported to be 86.6% (unpublished data). In this randomized clinical trial, we had two patients (14.2%) who died during our follow-up period within the local HTK group (mean follow-up duration of 344 days), which is similar to our documented survival rates among adult liver recipients. In the group that received our locally produced HTK solution, one patient died due to PNF. The patient received the locally produced HTK during his primary transplantation and received the Custodiol® HTK solution at re-transplantation. The disparity in death rates between the two groups was primarily due to the short follow-up and study design. However, considering the main goal of the study, we did not record any difference in primary and secondary clinical outcomes between the commercially available HTK solution and our locally produced solution.
One of the most important factors believed to affect patient outcomes are donor characteristics. In our study, donor baseline characteristics and type of organ donation (deceased or living) were similar between the comparison groups. The cold and warm ischemic times were similar in both groups, minimizing any bias related to organ selection and transplantation between the two comparison groups.
Since the first preservation solution was introduced, other countries and institutions have developed different formulations of preservation solutions for use in organ transplantation.10-12,22 Our locally produced HTK product is much cheaper and more available (for our region) than the commercially available HTK solutions. Considering the existing limitations in facilities in our region, if these results are confirmed in larger studies, this solution can be used as a substitute for the existing HTK products. This is especially important in middle- and low-income countries that have limited access to preservation solutions.
This study was not without limitations. Firstly, due to limitation in resources, we were not able to produce a large quantity of the local HTK fluid; as a result, this study was conducted as a pilot study and a larger study in the future will establish the exact efficacy and side effects of the locally produced HTK solution. Accordingly, other methods of statistical analysis would help to definitely conclude if the two products are equivalent. This study was primarily conducted in an adult population, and future studies should also be conducted among pediatric patients undergoing liver transplantations. Considering the short-term effects of the preservation solution on the organ of transplantation and the patient, we only had short-term outcomes set as our primary and secondary goals. The locally produced HTK solution was only evaluated for liver transplantations and other organs will be studied in the future. To the best of the authors’ knowledge, this is the first locally produced preservation product for organ transplantation in Iran.
In conclusion, based on the obtained results with the current sample size in this pilot study, no statistically significant difference was found between our locally produced HTK solution and the commercially available HTK product (Custodiol®) regarding clinical outcomes.
Acknowledgements
Authors would like to thank all the participant who accepted to be a part of this study.
Conflict of Interest Disclosures
The following authors are involved in PharMedCina Co.; SAM, YG, JR, RA, SK, MN, AS, SG, and SN.
Ethical Statement
All the study protocol followed the guideline of the declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Shiraz University of Medical Sciences, Shiraz, Iran (ethics code: IR.SUMS.REC.1398.626). This clinical trial was registered with the clinical trial registry at IRCT.ir (trial Id. 47439).
References
-
ONT-WHO. Global Observatory on Dontation and Transplantation. Available from: http://www.transplant-observatory.org/. Accessed March 2, 2021.
- Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez JV, Fuller BJ. Organ preservation: current concepts and new strategies for the next decade. Transfus Med Hemother 2011; 38(2):125-42. doi: 10.1159/000327033 [Crossref] [ Google Scholar]
- Fuller B, Froghi F, Davidson B. Organ preservation solutions: linking pharmacology to survival for the donor organ pathway. Curr Opin Organ Transplant 2018; 23(3):361-8. doi: 10.1097/mot.0000000000000525 [Crossref] [ Google Scholar]
- Jing L, Yao L, Zhao M, Peng LP, Liu M. Organ preservation: from the past to the future. Acta Pharmacol Sin 2018; 39(5):845-57. doi: 10.1038/aps.2017.182 [Crossref] [ Google Scholar]
- Belzer FO, Kalayoglu M, D’Alessandro AM, Pirsch JD, Sollinger HW, Hoffmann R. Organ preservation: experience with University of Wisconsin solution and plans for the future. Clin Transplant 1990; 4(2):73-7. [ Google Scholar]
- Parsons RF, Guarrera JV. Preservation solutions for static cold storage of abdominal allografts: which is best?. Curr Opin Organ Transplant 2014; 19(2):100-7. doi: 10.1097/mot.0000000000000063 [Crossref] [ Google Scholar]
- Karakoyun R, Romano A, Nordström J, Ericzon BG, Nowak G. Type of preservation solution, UW or HTK, has an impact on the incidence of biliary stricture following liver transplantation: a retrospective study. J Transplant 2019; 2019:8150736. doi: 10.1155/2019/8150736 [Crossref] [ Google Scholar]
- Schneeberger S, Biebl M, Steurer W, Hesse UJ, Troisi R, Langrehr JM. A prospective randomized multicenter trial comparing histidine-tryptophane-ketoglutarate versus University of Wisconsin perfusion solution in clinical pancreas transplantation. Transpl Int 2009; 22(2):217-24. doi: 10.1111/j.1432-2277.2008.00773.x [Crossref] [ Google Scholar]
- Krishnan H, Hannon MF, Bawa SM, Talbot D, Mirza D, Manas D. Comparison of the efficacy of University of Wisconsin solution and Newcastle organ perfusion fluid in the preservation of livers for transplantation. Transpl Int 1998; 11 Suppl 1:S387-9. doi: 10.1007/s001470050504 [Crossref] [ Google Scholar]
- Chen F, Nakamura T, Wada H. Development of new organ preservation solutions in Kyoto University. Yonsei Med J 2004; 45(6):1107-14. doi: 10.3349/ymj.2004.45.6.1107 [Crossref] [ Google Scholar]
- Mohr A, Brockmann JG, Becker F. HTK-N: modified histidine-tryptophan-ketoglutarate solution-a promising new tool in solid organ preservation. Int J Mol Sci 2020; 21(18):6468. doi: 10.3390/ijms21186468 [Crossref] [ Google Scholar]
- Wang S, Gumpper K, Tan T, Luo X, Guo H, Ming C. A novel organ preservation solution with efficient clearance of red blood cells improves kidney transplantation in a canine model. Cell Biosci 2018; 8:28. doi: 10.1186/s13578-018-0226-2 [Crossref] [ Google Scholar]
- Pirenne J, Van Gelder F, Coosemans W, Aerts R, Gunson B, Koshiba T. Type of donor aortic preservation solution and not cold ischemia time is a major determinant of biliary strictures after liver transplantation. Liver Transpl 2001; 7(6):540-5. doi: 10.1053/jlts.2001.24641 [Crossref] [ Google Scholar]
- Meine MH, Leipnitz I, Zanotelli ML, Schlindwein ES, Kiss G, Martini J. Comparison between IGL-1 and HTK preservation solutions in deceased donor liver transplantation. Transplant Proc 2015; 47(4):888-93. doi: 10.1016/j.transproceed.2015.03.033 [Crossref] [ Google Scholar]
- Nardo B, Bertelli R, Montalti R, Beltempo P, Puviani L, Pacilè V. Preliminary results of a clinical randomized study comparing Celsior and HTK solutions in liver preservation for transplantation. Transplant Proc 2005; 37(1):320-2. doi: 10.1016/j.transproceed.2004.11.028 [Crossref] [ Google Scholar]
- Wiederkehr JC, Igreja MR, Nogara MS, Goncalves N, Montemezzo GP, Wiederkehr HA. Use of IGL-1 preservation solution in liver transplantation. Transplant Proc 2014; 46(6):1809-11. doi: 10.1016/j.transproceed.2014.05.040 [Crossref] [ Google Scholar]
- Erhard J, Lange R, Scherer R, Kox WJ, Bretschneider HJ, Gebhard MM. Comparison of histidine-tryptophan-ketoglutarate (HTK) solution versus University of Wisconsin (UW) solution for organ preservation in human liver transplantation. A prospective, randomized study. Transpl Int 1994; 7(3):177-81. doi: 10.1007/bf00327084 [Crossref] [ Google Scholar]
- Rayya F, Harms J, Martin AP, Bartels M, Hauss J, Fangmann J. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in adult liver transplantation. Transplant Proc 2008; 40(4):891-4. doi: 10.1016/j.transproceed.2008.03.044 [Crossref] [ Google Scholar]
- Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transpl 2008; 14(3):365-73. doi: 10.1002/lt.21372 [Crossref] [ Google Scholar]
- Meine MH, Zanotelli ML, Neumann J, Kiss G, de Jesus Grezzana T, Leipnitz I. Randomized clinical assay for hepatic grafts preservation with University of Wisconsin or histidine-tryptophan-ketoglutarate solutions in liver transplantation. Transplant Proc 2006; 38(6):1872-5. doi: 10.1016/j.transproceed.2006.06.071 [Crossref] [ Google Scholar]
- Lock JF, Schwabauer E, Martus P, Videv N, Pratschke J, Malinowski M. Early diagnosis of primary nonfunction and indication for reoperation after liver transplantation. Liver Transpl 2010; 16(2):172-80. doi: 10.1002/lt.21973 [Crossref] [ Google Scholar]
- Büyük B, Demirci T, Adalı Y, Eroğlu HA. A new organ preservation solution for static cold storage of the liver Amniotic fluid. Acta Cir Bras 2019; 34(4):e201900402. doi: 10.1590/s0102-865020190040000002 [Crossref] [ Google Scholar]