Arch Iran Med. 25(1):17-25.
doi: 10.34172/aim.2022.04
Original Article
Outcomes of COVID-19 in Patients with Inflammatory Bowel Disease: Comparison with Household Members and the Role of IBD Medications
Ali Reza Sima 1  , Bahar Saberzadeh-Ardestani 1, Homayoon Vahedi 1, Hafez Fakheri 2, Fariborz Mansour-Ghanaei 3, Iradj Maleki 2, Siavosh Nasseri-Moghaddam 1, Hasan Vosoghinia 4, Mohammad Reza Ghadir 5, Ahmad Hormati 5, 6, Amir Kasaeian 1, 7, Amir Reza Radmard 8, Bardia Khosravi 1, Masoud Malekzadeh 1, Sudabeh Alatab 1, Anahita Sadeghi 1, Nayyereh Aminisani 9, Hossein Poustchi 1, Elnaz Gonoudi 1, Amir Anushiravani 1, Maryam Rayatpisheh 1, Jean-Frederic Colombel 10, Ryan C. Ungaro 10, *
, Bahar Saberzadeh-Ardestani 1, Homayoon Vahedi 1, Hafez Fakheri 2, Fariborz Mansour-Ghanaei 3, Iradj Maleki 2, Siavosh Nasseri-Moghaddam 1, Hasan Vosoghinia 4, Mohammad Reza Ghadir 5, Ahmad Hormati 5, 6, Amir Kasaeian 1, 7, Amir Reza Radmard 8, Bardia Khosravi 1, Masoud Malekzadeh 1, Sudabeh Alatab 1, Anahita Sadeghi 1, Nayyereh Aminisani 9, Hossein Poustchi 1, Elnaz Gonoudi 1, Amir Anushiravani 1, Maryam Rayatpisheh 1, Jean-Frederic Colombel 10, Ryan C. Ungaro 10, *  , Reza Malekzadeh 1, *
, Reza Malekzadeh 1, * 
Author information:
1Digestive Disease Research Center, Digestive Disease Research Institute, Tehran University of Medical Sciences, Tehran, Iran
2Gut and Liver Research Center, Mazandaran University of Medical Sciences, Sari, Iran
3Gastrointestinal and Liver Diseases Research Center, Guilan University of Medical Sciences, Rasht, Iran
4Gastroenterology and Hematology Department, Faculty of Medicine, Ghaem Hospital, Mashhad, Iran
5Gastroenterology and Hepatology Diseases Research Center, Qom University of Medical Science, Qom, Iran
6Gastrointestinal and Liver Diseases Research Center, Iran University of Medical Sciences, Tehran, Iran
7Hematology, Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran
8Department of Radiology, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
9Department of Epidemiology and Statistics, Faculty of Health Sciences, Neyshabur University of Medical Sciences, Neyshabur, Iran
10The Henry D. Janowitz Division of Gastroenterology Icahn School of Medicine at Mount Sinai, New York, USA
*Corresponding Authors: Ryan C. Ungaro MD, MS; Assistant Professor of Medicine, The Henry D. Janowitz Division of Gastroenterology Icahn School of Medicine at Mount Sinai, 17 E 102nd St 5th Floor, New York, NY 10029, USA. Tel: 212-241-4514, Email:
Ryan.ungaro@mssm.edu; Reza Malekzadeh, MD, AGAF; Professor of Medicine, Director Digestive Disease Research Institute, Tehran University of medical Sciences, Shariati hospital Postal Code: 14117-13135, Tel: + 98 (21) 824-15555, E-mail:
dr.reza.malekzadeh@gmail.com;
malek@tums.ac.ir
Abstract
Background:
Most data on the effect of inflammatory bowel disease (IBD) and its treatments on coronavirus disease 2019 (COVID-19) outcomes have not had non-IBD comparators. Hence, we aimed to describe COVID-19 outcomes in IBD compared to non-IBD patients.
Methods:
We conducted a prospective cohort study of registered IBD patients with confirmed COVID-19 from six provinces in Iran from February to April 2020. Proven COVID-19 patients were followed up at four weeks and the frequency of outcomes was assessed. Multivariable logistic regression was used to assess associations between demographics, clinical characteristics and COVID-19 outcomes.
Results:
Overall, 2159 IBD patients and 4721 household members were enrolled, with 84 (3.9%) and 49 (1.1%) participants having confirmed COVID-19, respectively. Household spread of COVID-19 was not common in this cohort (1.2%). While hospitalization was significantly more frequent in IBD patients compared with non-IBD household members (27.1% vs. 6.0%, P = 0.002), there was no significant difference in the frequency of severe cases. Age and presence of IBD were positively associated with hospitalization in IBD compared with non-IBD household members (OR: 1.06, 95% CI: 1.03-1.10; OR: 5.7, 95% CI: 2.02– 16.07, respectively). Age, presence of new gastrointestinal symptoms, and 5-aminosalicylic acid (5-ASA) use were associated with higher hospitalization rate in IBD patients (OR: 1.13, 95% CI: 1.05–1.23; OR: 6.49, 95% CI: 1.87–22.54; OR: 6.22, 95% CI: 1.90–20.36, respectively). Anti-tumor necrosis factor (TNF) was not associated with more severe outcomes.
Conclusion:
Age, presence of new gastrointestinal symptoms and use of 5-ASA were associated with increased hospitalization rate among IBD patients, while anti-TNF therapy had no statistical association.
Keywords: COVID-19, Inflammatory bowel disease, IBD medication
Copyright and License Information
© 2022 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Sima AR, Saberzadeh-Ardestani B, Vahedi H, Fakheri H, Mansour-Ghanaei F, Maleki I, et al. Outcomes of covid-19 in patients with inflammatory bowel disease: comparison with household members and the role of ibd medications. Arch Iran Med. 2022;25(1):17-25. doi: 10.34172/aim.2022.04
Introduction
Inflammatory bowel diseases (IBDs), comprising Crohn’s disease (CD) and ulcerative colitis (UC), lead to chronic inflammation of the gastrointestinal (GI) tract.1 It is estimated that IBD affects up to 6.8 million of the world population.1 Immune system dysregulation plays a key role in the disease pathogenesis.2 Therefore, immunosuppressive therapies such as azathioprine and corticosteroids are frequently used treatments for IBD patients,3 making them prone to infections.4-6
Case-fatality rates ranging from 2.3%–7.2% have been reported for coronavirus disease 2019 (COVID-19) since its emergence.7,8 Older age, male sex, and multiple comorbidities have been associated with severe outcomes.7-9 Patients with IBD and their physicians have had concerns about IBD routine care and the effect of IBD and related treatments on COVID-19 susceptibility and outcomes.10,11 IBD symptoms and COVID-19 may overlap since COVID-19 infection can manifest with gastrointestinal symptoms such as diarrhea.12,13 A number of studies have found that the COVID-19 incidence rate seems to be equal to or lower among IBD patients.14,15 Age, uncontrolled IBD, and the number of comorbidities have been associated with adverse outcomes in patients with IBD and COVID-19.16,17 Viral infections, such as Cytomegalovirus and Herpes Simplex Virus, can lead to more severe complications in patients with IBD taking immunosuppressive drugs.18 Less is known about the impact of immunosuppressive drugs on COVID-19 in IBD patients.
While immunosuppressive therapy could facilitate the activity of opportunistic organisms in the host,19 it may reduce severe outcomes in COVID-19 patients by inhibition of cytokine storm.20,21 Although some studies have suggested a positive association between IBD medications and severe COVID-19 outcomes, others have not seen such an association.16,17 Studies have shown that immunosuppressive therapies are not associated with adverse outcomes of previous coronaviruses.22 For better management of patients with IBD during the COVID-19 outbreak, a deeper understanding of the relationship between COVID-19 outcomes and IBD treatments is necessary. Most of the previous literature did not compare IBD patients to patients with similar exposures to COVID-19, such as their household members. This study aims to describe the characteristics of COVID-19 among IBD patients as compared to non-IBD household members and investigate the association between COVID-19 outcome and IBD treatments.
Materials and Methods
Study Design and Participants
In a multicenter prospective study from six provinces in Iran, recruited from February until April 2020, all IBD patients 18 years and older were enrolled in this study. The six included provinces represent the highest IBD prevalence registered in the Iranian Registry of Crohn’s and Colitis (IRCC) and these data are a subset of the IRCC.23,24 All patients were contacted by phone, and the following data were obtained: medical history and COVID-19 status among patients with IBD. They were also asked about all their household members with regard to COVID-19 and their health status. Age, sex, comorbidities, cigarette or waterpipe smoking, symptoms and outcomes of COVID-19 were collected from non-IBD household members. We used household members of patients with IBD to compare COVID-19 patients with and without IBD since their exposures were relatively similar. All IBD patients, calling the national hotline specified for IBD patients for COVID-19 during this period, were also enrolled. Figure 1 shows the study flow chart.
COVID-19 status was classified as suspected or confirmed. Patients with a history of contact with an infected person and a minimum of three signs/symptoms (fever, cough, dyspnea, dysosmia, and dysgeusia, or lung CT findings consistent with COVID-19) were considered as a suspected case.25,26 Data from patients with suspected COVID-19 were reviewed by an expert team of radiologists and gastroenterologists and based on that, the COVID-19 diagnosis was confirmed for each COVID-19 case. In addition to the above mentioned COVID-19 cases, patients with positive SARS-CoV-2 nasopharyngeal RT-PCR were considered as confirmed COVID-19 cases.
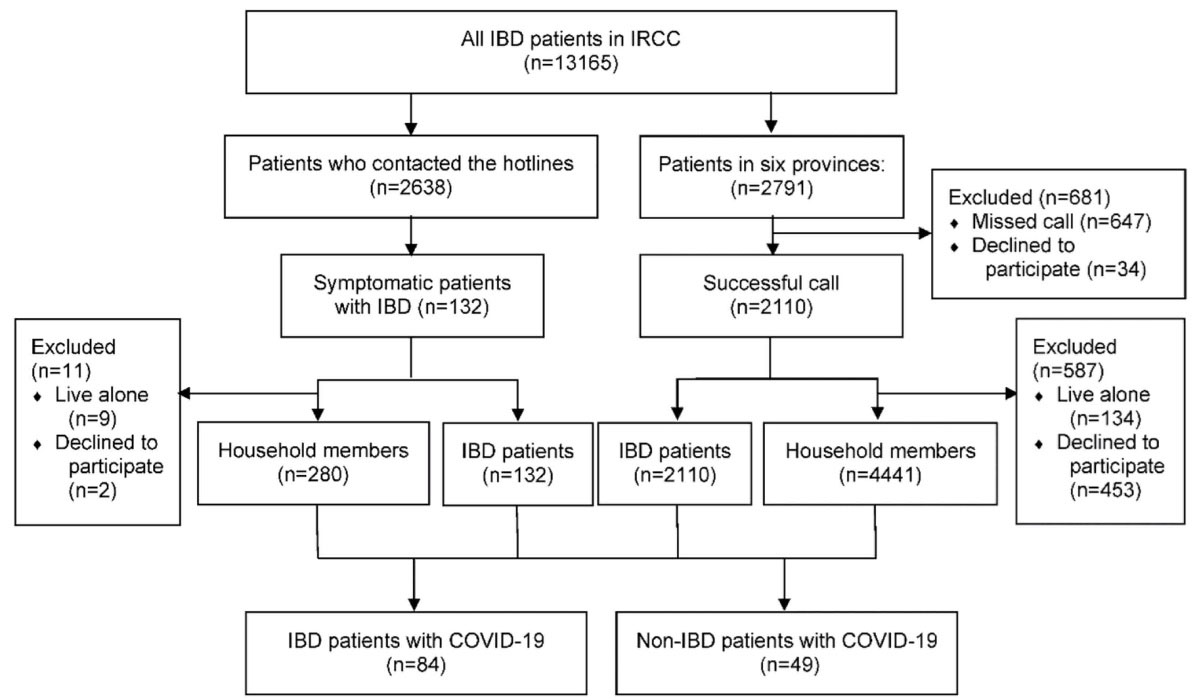
Figure 1.
Flow Diagram of Participant Screening and Enrollment. 2791 IBD patients were registered in six provinces in the Registry of Crohn’s and Colitis. 2110 patients answered our call and cooperated (response rate: 75.6%). 134 IBD patients lived alone and 453 did not cooperate to share their household members’ information; totally 4441 households were enrolled. Also, 2638 IBD patients had contacted our hotline with questions regarding COVID-19 and their IBD. 132 patients reported suspicious symptoms for COVID-19. The total number of IBD patients from the hotline and IRCC cohort with confirmed COVID-19 summed up to 84.
.
Flow Diagram of Participant Screening and Enrollment. 2791 IBD patients were registered in six provinces in the Registry of Crohn’s and Colitis. 2110 patients answered our call and cooperated (response rate: 75.6%). 134 IBD patients lived alone and 453 did not cooperate to share their household members’ information; totally 4441 households were enrolled. Also, 2638 IBD patients had contacted our hotline with questions regarding COVID-19 and their IBD. 132 patients reported suspicious symptoms for COVID-19. The total number of IBD patients from the hotline and IRCC cohort with confirmed COVID-19 summed up to 84.
Demographic characteristics and past medical history of enrolled patients were extracted from the IRCC database. We assessed the following for COVID-19 cases: hospitalization, intensive care unit (ICU) admission, respiratory support, and death at four weeks by telephone calls and medical record review.
Measurements
We gathered data on age, sex, cigarette or waterpipe smoking, diabetes mellitus, ischemic heart disease, chronic kidney disease, hypertension, cancer, chronic obstructive pulmonary disease (COPD), asthma, chronic liver disease, IBD disease type, IBD medications (i.e., 5-aminosalicylic acid (5-ASA), anti-tumor necrosis factor (anti-TNF), azathioprine/methotrexate/mercaptopurine, anti-TNF + azathioprine/methotrexate and corticosteroids), IBD activity (classified as remission, mild, moderate, severe), new GI symptoms (including abdominal pain, diarrhea, nausea, vomiting), dyspnea, myalgia, fever, cough, sore throat, headache, anorexia, SARS-CoV-2 RT-PCR, lung CT scan, emergency room visit, hospital length of stay, hospitalization, any event of ventilatory support, ICU admission, and death. Patients who used azathioprine or methotrexate or mercaptopurine were described as the group with immune modulator monotherapy and usage of anti-TNF with azathioprine or methotrexate was defined as combination therapy.
The primary outcome was hospitalization due to COIVD-19. Secondary outcomes included ICU admission, intubation and death. Severe COVID-19 was defined as any case of ICU admission, intubation, or death.
Statistical Analysis
Continuous variables are described as mean (standard deviation, SD) or median (interquartile range, IQR), for the variables without normal distribution. Categorical variables are represented as frequencies. One-way analysis of variance (ANOVA test), Kruskal-Wallis test, Bonferroni multiple-comparison test, and Mann-Whitney test were used for comparing means. Pearson chi-square and Fisher’s exact test (if needed) were used to compare categorical variables. Crude data are presented for the overall study population with multiple outcomes stratified by the patients’ characteristics. Univariable logistic analysis was performed for variables with a P value < 0.05 in the tests for comparing means of continuous variables and distribution of categorical variables. Variables with a P value < 0.05 in univariable logistic analysis that were congruent with prior evidence were included in a multivariable logistic regression model to investigate the independent effect of variables on hospitalization.
Also, we analyzed the association between the outcome of hospitalization and patients’ characteristics, including IBD medications, among patients with IBD using multivariable logistic regression.
We performed all statistical analyses using Stata 11.2 for Windows (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP). For follow-up pairwise comparisons with the Man-Whitney test, a P value < 0.016 was considered significant; otherwise, a P value < 0.05 was considered significant.
Results
Table 1 details the patients’ demographic and clinical characteristics. We collected 133 patients with COVID-19, which consisted of 84 IBD patients and 49 household controls. Patients who contacted the hotline comprised 54.7% of IBD patients with COVID-19 There were no significant age and sex differences between patients with and without IBD (P = 0.98 and 0.89, respectively). In the overall population, hypertension and diabetes were the most prevalent comorbidities, and the most commonly reported symptoms were dyspnea, cough, and fever. Dyspnea and cough were significantly more common among patients with IBD. Sixty percent of all IBD patients (38 patients out of 63) and 30% of household controls (13 patients out of 43) developed new GI symptoms (P = 0.002). Also, 1.2% of IBD patients with COVID-19 had household members with COVID-19.
Table 1.
Patients’ Demographic and Clinical Characteristics
|
Variable
sa, b |
N
133
|
Patients with IBD
n
=84
|
Patients without IBD
n
=49
|
P
Value
|
| Age, mean (SD) |
133 |
43.35 (14.1) |
43.30 (17.3) |
0.98 |
| Gender, n(%) |
133 |
|
|
0.89 |
| Female |
|
35 (41.6) |
21 (42.8) |
|
| Male |
|
49 (58.4) |
28 (57.2) |
|
| Comorbidities, n(%) |
133 |
|
|
|
| Hypertension |
|
11 (13.1) |
3 (6.1) |
0.25 |
| Chronic liver disease |
|
8 (9.5) |
0 (0) |
0.03* |
| Diabetes |
|
7 (8.3) |
2 (4.1) |
0.48 |
| COPD |
|
6 (7.1) |
2 (4.1) |
0.71 |
| Ischemic heart disease |
|
5 (5.9) |
3 (6.1) |
1.00 |
| Chronic kidney disease |
|
4 (4.7) |
2 (4.1) |
1.00 |
| Asthma |
|
2 (2.4) |
0 (0) |
0.53 |
| Cancer |
|
0 (0) |
0 (0) |
N/A |
| History of stroke |
|
0 (0) |
0 (0) |
N/A |
| Number of comorbidities |
133 |
|
|
0.32 |
| None |
|
50 (59.6) |
37 (75.5) |
|
| 1 |
|
22 (26.2) |
8 (16.3) |
|
| 2 |
|
6 (7.1) |
2 (4.1) |
|
| 3 |
|
6 (7.1) |
2 (4.1) |
|
| Cigarette smoking, n(%) |
133 |
2 (2.3) |
5 (10.2) |
0.49 |
| Waterpipe smoking, n(%) |
133 |
1 (1.2) |
2 (4.1) |
0.55 |
| Disease type, n(%) |
84 |
|
N/A |
N/A |
| CD |
|
24 (28.6) |
|
|
| UC |
|
60 (71.4) |
|
|
| IBD disease activity, n(%) |
84 |
|
N/A |
N/A |
| Remission |
|
45 (53.6) |
|
|
| Mild |
|
16 (19.1) |
|
|
| Moderate |
|
14 (16.8) |
|
|
| Severe |
|
9 (10.6) |
|
|
| IBD medication, n(%) |
133 |
|
N/A |
N/A |
| 5-ASA |
|
59 (53.6) |
|
|
| Anti-TNF monotherapy |
|
20 (15) |
|
|
| Immunomodulator monotherapy |
|
28 (21) |
|
|
| Combination therapy |
|
11 (8.3) |
|
|
| Corticosteroids |
|
13 (9.8) |
|
|
| Jak inhibitor |
|
0 (0) |
|
|
| COVID cases, n(%) |
133 |
|
|
0.530 |
| Suspected |
|
45 (53.6) |
29 (59.2) |
|
| Confirmed |
|
39 (46.4) |
20 (40.8) |
|
| New GI symptoms, n(%) |
106c |
38 (60.0) |
13 (30.0) |
0.002* |
| Symptoms, n(%) |
133 |
|
|
|
| None |
|
3 (3.6) |
5 (10.2) |
0.14 |
| Dyspnea |
|
64 (76.2) |
26 (53.1) |
0.007* |
| Myalgia |
|
24 (28.6) |
10 (20.4) |
0.29 |
| Fever |
|
33 (39.3) |
21 (42.8) |
0.68 |
| Chills |
|
36 (27.1) |
12 (24.5) |
0.03 |
| Cough |
|
45 (42.9) |
11 (22.4) |
< 0.001* |
| Sore throat |
|
21 (25.0) |
6 (12.2) |
0.78 |
| Headache |
|
5 (5.6) |
2 (4.1) |
1.00 |
| Anorexia |
|
1 (1.2) |
0 (0) |
1.00 |
| Emergency room visit, n(%) |
133 |
33 (39.3) |
11 (22.4) |
0.04* |
| Hospital length of stay in days, mean (SD) |
133 |
3.21 (5.7) |
1.20 (2.9) |
0.46 |
| Hospitalization, n(%) |
133 |
36 (42.9) |
8 (16.3) |
0.002* |
| Intubation, n(%) |
133 |
4 (4.8) |
2 (4.1) |
1 |
| ICU admission, n(%) |
133 |
7 (8.3) |
2 (4.1) |
0.48 |
| Death during hospitalization, n(%) |
133 |
1 (1.2) |
1 (2.0) |
1 |
COPD, chronic obstructive pulmonary disease; 5-ASA, 5-aminosalicylic acid; TNF, tumor necrosis factor; JAK, Janus kinase; CT scan, computed tomography scan; ICU, intensive care unit; IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; GI, gastrointestinal.
aPercentages do not include missing values and were calculated for each row by dividing on the corresponding N value.
bPercentages from each subcategory may not add up to the exact number of total reported cases due to missing values and/or non-mutually exclusive variables.
c New GI symptoms value was missing in 21 patients with IBD and 6 patients without IBD.
*Statistically significant.
Totally, 71.4% of patients with IBD had UC, and more than 50% of patients with IBD were in remission. The most commonly used medication was 5-aminosalicylic acid (5-ASA) (70.24%). Other medications are summarized in Table 1.
The distribution of different outcomes by demographic and clinical data in the overall study population (COVID-19 patients with and without IBD) are presented in Table 2. Thirty-six IBD patients (42.86%) and eight household members (16.33) needed hospitalization (P = 0.002), with 7 (8.33%) and 2 (4.08%) patients admitted to the ICU, respectively (P = 0.484). While four IBD patients (4.76%) and two household members (4.08%) were intubated, only one patient died in each group.
Table 2.
Distribution of Different Outcomes by Demographic and Clinical Data in Overall Study Population
|
Variables
a
|
N=
133
|
Hospitalization,
n
(%)
|
Hospital Length of Stays in Days,
Median (IQR)
|
ICU Admission,
n
(%)
|
Intubation,
n
(%)
|
Death,
n
(%)
|
ICU or Intubation or Death,
n
(%)
|
|
Age
|
|
|
|
|
|
|
|
| 18–29 |
26 |
5 (19.2) |
7 (5–7) |
0 |
0 |
0 (0) |
0 (0) |
| 30–39 |
34 |
5 (14.7) |
6 (5–6) |
0 |
0 |
0 (0) |
0 (0) |
| 40–49 |
34 |
13 (38.2) |
6.5 (3.5-10) |
2 (5.9) |
0 |
0 (0) |
2 (5.9) |
| 50–59 |
17 |
7 (41.2) |
6 (3–7) |
2 (11.8) |
0 |
1 (5.9) |
2 (11.8) |
| 60–69 |
15 |
8 (53.3) |
6 (4.5–11) |
3 (20) |
3 (20) |
0 (0) |
5 (33.3) |
| 70–79 |
3 |
3 (100) |
9 (5–9) |
2 (66.7) |
2 (66.7) |
0 (0) |
2 (66.7) |
| ≥ 80 |
4 |
3 (75) |
5 (5–12) |
0 (0) |
1 (25) |
1 (25) |
2 (50) |
|
P value |
- |
0.002* |
0.929 |
0.001* |
< 0.001* |
0.028* |
< 0.001* |
|
Gender
|
|
|
|
|
|
|
|
| Female |
56 |
18 (32.1) |
6.5 (5–8) |
5 (8.9) |
5 (8.9) |
0 (0) |
8 (14.3) |
| Male |
77 |
26 (33.8) |
6 (4–8) |
4 (5.2) |
1 (1.3) |
2 (2.6) |
5 (6.5) |
|
P value |
- |
1 |
0.060 |
0.492 |
0.082 |
0.509 |
0.151 |
|
Number of comorbidities
|
|
|
|
|
|
|
|
| ≤ 2 |
125 |
38 (86.4) |
0 (0–4) |
5 (55.6) |
4 (66.7) |
1 (50.0) |
8 (61.5) |
| > 2 |
8 |
6 (13.4) |
8.5 (1.5–10.5) |
8 (44.4) |
2 (33.3) |
1 (50.0) |
5 (38.46) |
|
P value |
- |
0.016* |
0.067 |
0.001* |
0.042* |
0.117 |
< 0.001* |
|
Smoking
|
|
|
|
|
|
|
|
| Not smoker |
126 |
42 (33.3) |
8 (8–8) |
9 (7.1) |
6 (4.8) |
2 (1.6) |
13 (10.3) |
| Smoker |
7 |
2 (28.6) |
6 (5–8) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
|
P value |
- |
0.794 |
0.440 |
1 |
1 |
1 |
1 |
|
IBD status
|
|
|
|
|
|
|
|
| Non-IBD |
49 |
8 (16.3) |
7 (5–8) |
2 (4.1) |
2 (4.1) |
1 (2.0) |
3 (6.1) |
| IBD patient |
84 |
36 (42.9) |
6 (4–8) |
7 (8.3) |
4 (4.8) |
1 (1.2) |
10 (11.9) |
|
P value |
- |
0.002* |
0.460 |
0.484 |
1 |
1 |
0.279 |
|
New GI symptoms
|
|
|
|
|
|
|
|
| Absent |
55b |
8 (14.6) |
5 (5–7) |
0 (0) |
2 (3.6) |
0 (0) |
2 (3.6) |
| Present |
51 |
24 (47.1) |
7 (5–9) |
7 (13.7) |
4 (7.8) |
1 (1.9) |
9 (17.7) |
|
P value |
- |
< 0.001* |
0.424 |
0.005* |
0.425 |
0.481 |
0.025* |
GI, Gastrointestinal; IBD, inflammatory bowel disease; ICU, intensive care unit.
aPercentages were calculated for each row by dividing on the corresponding N value.
b Total number of New GI symptoms’ observation was 106.
*Statistically significant.
Univariable analysis showed a significant association between the primary outcome of hospitalization and older age, number of comorbidities more than two, presence of IBD, and new GI symptoms (Table 2). Multivariable logistic regression of the overall study population showed a significant association between age and presence of IBD with hospitalization (Table 3). While each additional year was associated with 6% higher chance of hospitalization (95% CI: 1.03–1.10, P < 0.001), the presence of IBD increased the odds of hospital admission by 5.70 times (95% CI: 2.02–16.07, P = 0.001).
Table 3.
Association of Hospitalization and Patients’ Characteristics Among COVID-19 Patients with and without IBD
|
Variable (Referent Group)
|
Univariate
|
Multivariate
|
Odds Ratio (95% CI)
(N=133)
|
P
Value
|
Odds Ratio (95% CI)
(N=133)
|
P
Value
|
| Age |
1.06 (1.03-1.09) |
< 0.001* |
1.06 (1.03-1.10) |
< 0.001* |
| Comorbidities (≤ 2) |
6.86 (1.32- 35.58) |
0.022* |
1.77(0.28-10.99) |
0.537 |
| IBD (No) |
3.84 (1.61-9.19) |
0.002* |
5.70 (2.02-16.07) |
0.001* |
IBD, inflammatory bowel disease.
*Statistically significant
Table 4 illustrates the distribution of different outcomes by IBD characteristics and medications. In the univariable analysis, 5-ASA usage was associated with more frequent hospitalization (P < 0.001). There was no significant association between other IBD medications (i.e., anti-TNF monotherapy, immune modulator monotherapy, combination therapy, and corticosteroids) and hospitalization among patients with IBD.
Table 4.
Distribution of Different Outcomes by IBD Characteristics and Medications in COVID-19 Patients with IBD
|
Variables
a
|
(N=
84)
|
Hospitalization,
n
(%)
|
Hospital Length of Stays in days,
median (IQR)
|
ICU Admission,
n
(%)
|
Intubation,
n
(%)
|
Death,
n
(%)
|
ICU or Intubation or Death,
n
(%)
|
|
Disease type
|
|
|
|
|
|
|
|
| CD |
24 |
8 (33.3) |
7 (5.5–9.5) |
1 (4.2) |
0 (0) |
0 (0) |
1 (4.2) |
| UC |
60 |
28 (46.7) |
5 (4-8) |
6 (10) |
4 (6.7) |
1 (1.7) |
9 (15) |
|
P value |
- |
0.265 |
0.555 |
0.667 |
0.321 |
1 |
0.268 |
|
Disease severity
|
|
|
|
|
|
|
|
| Remission |
45 |
16 (35.6) |
6 (5–7) |
1 (2.2) |
3 (6.7) |
1 (2.2) |
4 (8.9) |
| Mild |
16 |
9 (56.3) |
8 (5–8) |
3 (18.8) |
0 (0) |
0 (0) |
3 (18.8) |
| Moderate |
14 |
9 (64.3) |
5 (4–8) |
2 (14.3) |
1 (7.2) |
0 (0) |
2 (14.3) |
| Severe |
9 |
2 (22.2) |
9 (1–17) |
1 (11.1) |
0 (0) |
0 (0) |
1 (11.1) |
|
P value |
- |
0.103 |
0.137 |
0.077 |
0.767 |
1 |
0.722 |
|
5-ASA
|
|
|
|
|
|
|
|
| IBD nonusers |
25 |
4 (16) |
7.5 (5–19) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
| IBD users |
59 |
32 (54.2) |
6 (4–8) |
7 (11.9) |
4 (6.8) |
1 (1.7) |
10 (16.9) |
|
P value |
- |
0.002* |
0.012* |
0.098 |
0.313 |
1 |
0.029* |
|
Anti-TNF monotherapy
|
|
|
|
|
|
|
|
| IBD nonusers |
64 |
30 (46.9) |
6 (4–8) |
4 (6.3) |
4 (6.3) |
1 (1.6) |
7 (10.9) |
| IBD users |
20 |
6 (30) |
8 (3–13) |
3 (15) |
0 (0) |
0 (0) |
3 (15) |
|
P value |
- |
0.183 |
0.337 |
0.349 |
0.568 |
1 |
0.695 |
|
Immunomodulator monotherapy
|
|
|
|
|
|
|
|
| IBD nonusers |
56 |
27 (48.2) |
6 (5–8) |
4 (7.1) |
4 (7.1) |
1 (1.8) |
7 (12.5) |
| IBD users |
28 |
9 (32.1) |
8 (3–9) |
3 (10.7) |
0 (0) |
0 (0) |
3 (10.7) |
|
P value |
- |
0.161 |
0.298 |
0.681 |
0.296 |
1 |
1 |
|
Combination therapy
|
|
|
|
|
|
|
|
| IBD nonusers |
73 |
32 (43.8) |
6 (4–8) |
5 (6.6) |
4 (5.5) |
1 (1.4) |
8 (10.9) |
| IBD users |
11 |
4 (36.4) |
5.5 (2–12.5) |
2 (18.2) |
0 (0) |
0 (0) |
2 (18.2) |
|
P value |
- |
0.751 |
0.671 |
0.227 |
1 |
1 |
0.613 |
|
Corticosteroids
|
|
|
|
|
|
|
|
| IBD nonusers |
71 |
28 (39.4) |
6 (5–8) |
5 (7.0) |
3 (4.2) |
1 (1.4) |
8 (11.3) |
| IBD users |
13 |
8 (61.5) |
4 (3–11) |
2 (15.4) |
1 (7.7) |
0 (0) |
2 (15.4) |
|
P value |
- |
0.139 |
0.528 |
0.295 |
0.496 |
1 |
0.649 |
5-ASA, 5-aminosalicylic acid; TNF, tumor necrosis factor; ICU, intensive care unit; IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis.
aPercentages were calculated for each row by dividing on the corresponding N value.
*Statistically significant.
The result of the subgroup analysis among patients with IBD is summarized in Table 5. When we analyzed the association of hospitalization and patients’ characteristics, the multivariable logistic regression revealed a significant association between age, new GI symptoms, and 5-ASA usage with the primary outcome of hospitalization among these patients (n = 84). While age was associated with 1.14 times higher odds of hospitalization (95% CI: 1.05–1.24, P = 0.002), the presence of new GI symptoms increased the odds of hospital admission by 17.74 times (95% CI: 2.07–91.30, P = 0.007). In addition, 5-ASA usage was associated with 9.30 times higher odds of hospitalization (95% CI: 1.35–63.87, P = 0.023).
Table 5.
Association of Hospitalization and Patients’ Characteristics, including IBD Medications among COVID-19 Patients with IBD
|
Variable (Referent group)
|
Univariate
|
Multivariate
|
Odds Ratio (95% CI)
(N=84)
|
P
Value
|
Odds Ratio (95% CI)
(N=84)
|
P
Value
|
| Age |
1.13 (1.05–1.23) |
0.002* |
1.14 (1.05–1.24) |
0.002* |
| Comorbidities (≤ 2) |
7.58 (0.84–68.03) |
0.070 |
- |
- |
| New GI symptoms (none) |
6.49 (1.87–22.54) |
0.003* |
17.74 (2.07–91.30) |
0.007* |
| 5-ASA (none) |
6.22 (1.90–20.36) |
0.003* |
9.30 (1.35–63.87) |
0.023* |
Abbreviations: GI, gastrointestinal; 5-ASA, 5-aminosalicylic acid; IBD, inflammatory bowel disease.
*Statistically significant.
Univariable analysis of the secondary outcomes revealed a significant positive association between age and multiple adverse outcomes such as ICU admission, intubation, and death. There was no significant difference between the frequency of severe cases in patients with and without IBD. Furthermore, older age, more than two comorbidities, presence of new GI symptoms and 5-ASA usage were associated with more frequent severe outcome (any case of being admitted to the ICU, intubated, or deceased) (Tables 2 and 4).
Discussion
In this study, we described the clinical characteristics and outcomes in COVID-19 patients with IBD and non-IBD household members. Since IBD patients and their household members had similar exposures, we investigated the risk factors of unfavorable outcomes by comparing these two groups. While hospitalization due to COVID-19 was significantly more frequent in IBD patients compared with non-IBD household members, there was no significant difference between the frequency of severe cases in patients with and without IBD. We observed that age, presence of new GI symptoms, and 5-ASA usage were associated with greater odds of hospitalization among patients with IBD. Anti-TNF use was not significantly related to hospitalization or severe outcomes among patients with IBD.
Hospitalization due to COVID-19 was significantly higher among patients with IBD in our study. As far as we know, this is the first time that the hospitalization frequency of patients with IBD is compared with non-IBD household members with similar exposures. This increased risk of hospitalization among IBD patients could be due to worsening of the IBD course or could be related to the COVID-19 course in IBD patients. Also, the baseline activity of IBD could have influenced these results; it is necessary to investigate this factor in future studies and further studies are needed to investigate causality. The 36% hospitalization and 11.2% severe cases with a 1.2% case fatality rate among patients with IBD is relatively similar to previous studies. Brenner et al reported 31% hospitalization, 7% severe COVID-19 and 3% case fatality rate among 525 cases of patients with IBD from 33 countries.17 A study on outcomes of COVID-19 in 79 patients with IBD in Italy by Bezzio et al showed 28% hospitalization, 3% endotracheal intubation, and 8% death.16 Also, Higgins et al mentioned that among 239 reported cases, 3% experienced ICU admission.14 In our study, there was no significant difference between the frequency of severe cases in patients with and without IBD. This finding is similar to the study by Lukin et al, who compared outcomes in COVID-19 patients with or without IBD using a matched cohort design. They reported a severity rate of 24% in IBD patients which was not significantly different from 35% among patients without IBD.27 Differences in the percentage of severe cases can be attributed to the discrepancy of definitions for disease severity across studies. Furthermore, due to the small event number of severe cases in our study, larger studies are necessary to compare the severity of COVID-19 between patients with and without IBD. Although dyspnea and cough were significantly more common among IBD patients, this could be attributed to recall bias as IBD patients provided information about their household members in our study.
Although Bezzio et al found a significant association between UC diagnosis and IBD activity and COVID-19 pneumonia16 and Lukin et al reported an association between diagnosis of UC (in comparison to CD) and emergency visit or admission (adjusted odds ratio = 12.7),27 we found no significant relationship between these variables and COVID-19 outcomes. This finding could be due to the limited number of patients with CD and severe outcomes in our study.
Age was associated with an increased risk of hospitalization and severe outcomes, and this is consistent with previous works. Based on the study by Brenner and Ungaro et al, increasing age is related to 1.04 higher odds of severe COVID-19.17 Also, Bezzio et al reported a significant association between age over 65 years and COVID-19 pneumonia.16 We found that the presence of more than two comorbidities was not significantly associated with hospitalization in multivariable analysis, whereas Brenner et al reported 2.9 higher adjusted odds of severe outcomes in patients with more than two comorbidities. This discrepancy may be due to our smaller sample size.
Sixty percent of patients with IBD experienced new GI symptoms, which was double the figure for patients without IBD. This fits well with the results of Lukin et al who found GI symptoms such as diarrhea and abdominal pain to be more frequent among patients with IBD (45 versus 19%, P < 0.001 and 20% versus 5%, P = 0.001, respectively).27 Also, new GI symptoms were significantly associated with hospitalization and severe outcomes. This is inconsistent with the findings of Lukin et al, who reported no association between emergency visit or admission and GI symptoms.27 This discrepancy could be attributed to reporting bias in our study or the GI symptoms could be interpreted as markers of IBD activity. Further studies are needed to explore the association between GI symptoms and IBD activity. Also, future studies should explore other possible explanations such as the effect of COVID-19 on IBD or the intestinal susceptibility of SARS-CoV-2 infection in each group.
5-ASA usage was significantly associated with hospitalization among IBD patients, and there was a positive association between 5-ASA and severe outcomes. This is consistent with previous studies that reported sulfasalazine or 5-ASA use related to 3.1 higher odds of severe COVID-19 (95% CI: 1.3–7.7).17 Ungaro et al reported a 3.52-time higher risk of severe COVID-19 for IBD patients with mesalamine/sulfasalazine usage, in comparison with anti-TNF monotherapy.28 This finding could be due to under-treatment of IBD and patients having active disease or an effect of the treatment itself. Future studies are needed to clarify this association.
According to our results, there was no significant association between anti-TNF use and hospitalization among patients with IBD. This result is in line with previous studies.17,27,29 Furthermore, Ungaro et al have reported the possibility of a relative protective effect for anti-TNF usage among patients with IBD.28 Lukin et al reported numerically higher emergency visits/hospitalizations among patients with IBD who did not receive biologic medications (P = 0.197).27
Although corticosteroid use was not associated with hospitalization or severe outcome in our study, this result should be interpreted with caution due to the small number of patients with prednisolone usage in our study population. This is consistent with the study by Bezzio et al,16 but is in contrast with the findings of Brenner et al17 Akiyama et al. reported increased risk of severe COVID-19 among patients with autoimmune disease who used glucocorticoids in comparison with anti-TNF monotherapy.30
The major strengths of our study include the use of a nationwide IRCC cohort for approaching patients with IBD and comparing them with their non-IBD household members with COVID-19. Also, we followed patients for four weeks, and the expert committee confirmed COVID-19 by individual chart review.
It is plausible that some limitations may have influenced the results obtained. To begin with, we enrolled patients who were able to pick up the phone and due to the limited sample size, we enrolled patients with IBD who have called the hotline; these could cause a selection bias. Furthermore, since the IBD patients answered the questions about themselves and their household members, this could lead to a reporting bias. Also, the investigation of rare risk factors, such as Janus kinase inhibitor usage, was not possible. An additional possible source of error is the underestimation of the disease as a result of silent carriers and our diagnostic limitations. Moreover, the household spread of COVID-19 which was low in this cohort could be a result of not testing for COVID-19 and silent carriers.
In conclusion, household spread of COVID-19 was not common in this cohort. New GI symptoms were more common in IBD patients than non-IBD household members. Hospitalization was significantly higher among patients with IBD in our study, and the evidence from this study suggests age, presence of new GI symptoms, and 5-ASA usage as risk factors of hospitalization among patients with IBD. There was no significant difference between the frequency of severe cases in patients with and without IBD. Finally, anti-TNF use was not significantly related to hospitalization or severe outcomes among patients with IBD. Further work needs to be done to establish whether IBD medications are associated with hospitalization or severe outcomes, especially in comparison to patients without IBD.
Acknowledgements
All authors contributed solely as volunteers. We are grateful to the employees of the IRCC cohort for their contribution to data gathering. We acknowledge all contributors in six provinces of Iran (Tehran, Alborz, Mazandaran, Gilan, Khorasan Razavi, Qom) who have reported IBD patients to IRCC.
IRCC members who contributed to this study:
AbdolRasool Hayatbakhsh M.D., Abdossamad Gharavi M.D., Afshin Shafaghi M.D., Ali Beheshti Namdar M.D., Amineh Hojati M.D., Amir Hossein Faraji M.D., Amirabbas Hasan Zadeh M.D., Arash Kazemi Veisari M.D., Elham Mokhtari Amirmajdi M.D., Fatemeh Farahmand M.D., Forough Alborzi M.D., Hasan Ali Metanat M.D., Hayedeh Adilipour M.D., Hosein Alimadadi M.D., Jalaluddin Naghshbandi M.D., Katrin Behzad M.D., Kourosh Mojtahedi M.D., Ladan Goshayeshi M.D., Mahdi Pezeshki Modares M.D., Mahmoud Hoseinian M.D., Mahmoud Yousefi Mashhour M.D., Masoud Dooghaie M.D., Moghadam M.D., Mehdi Saberi Firoozi M.D., Mehri Najafi M.D., Mitra Ahadi M.D., Mohammad Reza Farzanehfar M.D., Nadieh Baniasadi M.D., Sahar Rismantab M.D., Sanaz Gonoodi M.D., Seyed Mohammad Valizadeh Toosi M.D., Tarang Taghvaei M.D., Vahid Hosseini M.D.
Authors’ Contribution
Conceptualization: all authors. Methodology: all authors. Formal analysis: AS, BAS, SNM, AK, BK, FC, RCU, RM. Funding acquisition: AS, RM. Project administration: AS. Visualization: AS, BSA. Writing - original draft: AS, BSA. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Conflict of Interest Disclosures
RCU has served as an advisory board member or consultant for Eli Lilly, Janssen, Pfizer, and Takeda; research support from AbbVie, Boehringer Ingelheim, and Pfizer. RCU funded by an NIH K23 Career Development Award (K23KD111995-01A1).
JFC: research grants from AbbVie, Janssen Pharmaceuticals and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Inc. Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Landos, Ipsen, Medimmune, Merck, Novartis, Pfizer, Shire, Takeda, Tigenix, Viela bio; and hold stock options in Intestinal Biotech Development and Genfit. For the remaining authors none were declared.
Ethical Statement
This study was approved by the ethics committee of Tehran University of Medical Sciences. Informed consent was obtained from the study participants. (IRB number: IR.TUMS.MEDICINE.REC.1399.514)
References
- Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020; 5(1):17-30. doi: 10.1016/s2468-1253(19)30333-4 [Crossref] [ Google Scholar]
- Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol 2014; 20(1):91-9. doi: 10.3748/wjg.v20.i1.91 [Crossref] [ Google Scholar]
- Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68(Suppl 3):s1-s106. doi: 10.1136/gutjnl-2019-318484 [Crossref] [ Google Scholar]
- Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014; 8(6):443-68. doi: 10.1016/j.crohns.2013.12.013 [Crossref] [ Google Scholar]
- Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017; 389(10080):1756-70. doi: 10.1016/s0140-6736(16)32126-2 [Crossref] [ Google Scholar]
- Beaugerie L, Kirchgesner J. Balancing benefit vs risk of immunosuppressive therapy for individual patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2019; 17(3):370-9. doi: 10.1016/j.cgh.2018.07.013 [Crossref] [ Google Scholar]
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020; 323(18):1775-6. doi: 10.1001/jama.2020.4683 [Crossref] [ Google Scholar]
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323(13):1239-42. doi: 10.1001/jama.2020.2648 [Crossref] [ Google Scholar]
- CDC COVID-19 Response Team. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep 2020; 69(13):382-6. doi: 10.15585/mmwr.mm6913e2 [Crossref] [ Google Scholar]
- Bodini G, Demarzo MG, Casagrande E, De Maria C, Kayali S, Ziola S. Concerns related to COVID-19 pandemic among patients with inflammatory bowel disease and its influence on patient management. Eur J Clin Invest 2020; 50(5):e13233. doi: 10.1111/eci.13233 [Crossref] [ Google Scholar]
- Chen Y, Yu Q, Farraye FA, Kochhar GS, Bernstein CN, Navaneethan U. Patterns of endoscopy during COVID-19 pandemic: a global survey of interventional inflammatory bowel disease practice. Intest Res 2021; 19(3):332-40. doi: 10.5217/ir.2020.00037 [Crossref] [ Google Scholar]
- Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol 2020; 115(5):766-73. doi: 10.14309/ajg.0000000000000620 [Crossref] [ Google Scholar]
- da Luz BB, de Oliveira NMT, Dos Santos IW, Paza LZ, de Mello Braga LL, da Silva Platner F. An overview of the gut side of the SARS-CoV-2 infection. Intest Res 2021; 19(4):379-85. doi: 10.5217/ir.2020.00087 [Crossref] [ Google Scholar]
- Higgins PDR, Ng S, Danese S, Rao K. The risk of SARS-CoV-2 in immunosuppressed IBD patients. Crohns Colitis 360 2020; 2(2):otaa026. doi: 10.1093/crocol/otaa026 [Crossref] [ Google Scholar]
- Gubatan J, Levitte S, Balabanis T, Patel A, Sharma A, Habtezion A. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology 2020; 159(3):1141-4. doi: 10.1053/j.gastro.2020.05.009 [Crossref] [ Google Scholar]
- Bezzio C, Saibeni S, Variola A, Allocca M, Massari A, Gerardi V. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut 2020; 69(7):1213-7. doi: 10.1136/gutjnl-2020-321411 [Crossref] [ Google Scholar]
- Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020; 159(2):481-91. doi: 10.1053/j.gastro.2020.05.032 [Crossref] [ Google Scholar]
- Yi F, Zhao J, Luckheeram RV, Lei Y, Wang C, Huang S. The prevalence and risk factors of cytomegalovirus infection in inflammatory bowel disease in Wuhan, Central China. Virol J 2013; 10:43. doi: 10.1186/1743-422x-10-43 [Crossref] [ Google Scholar]
- Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology 2018; 155(2):337-46. doi: 10.1053/j.gastro.2018.04.012 [Crossref] [ Google Scholar]
- Zhang X, Song K, Tong F, Fei M, Guo H, Lu Z. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv 2020; 4(7):1307-10. doi: 10.1182/bloodadvances.2020001907 [Crossref] [ Google Scholar]
- Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol 2020; 31(7):961-4. doi: 10.1016/j.annonc.2020.03.300 [Crossref] [ Google Scholar]
- D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl 2020; 26(6):832-4. doi: 10.1002/lt.25756 [Crossref] [ Google Scholar]
- Malekzadeh MM, Sima A, Alatab S, Sadeghi A, Ebrahimi Daryani N, Adibi P. Iranian Registry of Crohn’s and Colitis: study profile of first nation-wide inflammatory bowel disease registry in Middle East. Intest Res 2019; 17(3):330-9. doi: 10.5217/ir.2018.00157 [Crossref] [ Google Scholar]
- Anushiravani A, Vahedi H, Fakheri H, Mansour-Ghanaei F, Maleki I, Nasseri-Moghaddam S. A supporting system for management of patients with inflammatory bowel disease during COVID-19 outbreak: Iranian experience-study protocol. Middle East J Dig Dis 2020; 12(4):238-45. doi: 10.34172/mejdd.2020.188 [Crossref] [ Google Scholar]
- Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 2020; 215(1):87-93. doi: 10.2214/ajr.20.23034 [Crossref] [ Google Scholar]
- World Health Organization (WHO). Coronavirus. Secondary coronavirus 2020. Available from: https://www.who.int/health-topics/coronavirus.
- Lukin DJ, Kumar A, Hajifathalian K, Sharaiha RZ, Scherl EJ, Longman RS. Baseline disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology 2020; 159(4):1541-4. doi: 10.1053/j.gastro.2020.05.066 [Crossref] [ Google Scholar]
- Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut 2021; 70(4):725-32. doi: 10.1136/gutjnl-2020-322539 [Crossref] [ Google Scholar]
- Khan N, Patel D, Xie D, Lewis J, Trivedi C, Yang YX. Impact of Anti-Tumor Necrosis Factor and Thiopurine Medications on the Development of COVID-19 in Patients with Inflammatory Bowel Disease: a Nationwide Veterans Administration Cohort Study. Gastroenterology 2020; 159(4):1545-6. doi: 10.1053/j.gastro.2020.05.065 [Crossref] [ Google Scholar]
- Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis 2020. doi: 10.1136/annrheumdis-2020-218946 [Crossref]