Arch Iran Med. 25(2):98-104.
doi: 10.34172/aim.2022.16
Original Article
Effect of Prophylactic Caffeine on Noninvasive Respiratory Support in Preterm Neonates Weighing 1250–2000 g: A Randomized Controlled Trial
Ramin Iranpour 1, *  , Amir-Mohammad Armanian 1, Nooshin Miladi 1, Awat Feizi 2
, Amir-Mohammad Armanian 1, Nooshin Miladi 1, Awat Feizi 2
Author information:
1Department of Pediatrics, Division of Neonatology Isfahan University of Medical Sciences, Isfahan, Iran
2Department of Biostatistics and Epidemiology, Isfahan University of Medical Sciences, Isfahan, Iran
*Corresponding Author: Ramin Iranpour, MD; Department of Pediatrics, Imam-Hossein children’s Hospital, Imam-Khomeini Blvd, Isfahan, Iran. Postal code: 8195163381, Fax: +983133868286, Tel: +983133868247, Mobile: +989131131653, Email:
ramin.iranpour@gmail.com
Abstract
Background:
Caffeine is commonly used to prevent or treat apnea in preterm neonates. The present trial was designed to determine the effect of caffeine on reducing the time required for nasal continuous positive airway pressure (NCPAP) in neonates with respiratory distress syndrome (RDS).
Methods:
In a randomized controlled trial, a total of 90 neonates (birth weight between 1250 and 2000 g) who were clinically diagnosed with RDS were subjected to random assignment to one of the two groups of caffeine (n=45) or control (n=45). Infants in the caffeine group received 20 mg/kg caffeine as the initial dose, and then 10 mg/kg daily as the maintenance dose. Infants in the control group did not receive any placebo or similar drugs. The primary outcome was the duration time of respiratory support with NCPAP.
Results:
The mean (SD) duration of NCPAP differed significantly and was shorter among the infants in the caffeine group than those assigned to the control group (41.53 (43.25) versus 78.48 (114.25) hours, respectively; mean difference: -36.95; 95%CI: -73.14, -0.76; P = 0.04). Apnea of prematurity (AOP) occurred in 2 (4.4%) newborns in the caffeine group and in 9 (20%) of the infants in the control condition [proportion difference: -15.6% (-29.8,-1.8); (P = 0.02)]. The incidence of intraventricular hemorrhage (IVH) was higher in the control group than in the caffeine group after one week (P = 0.03). The incidence of chronic lung disease (CLD), infection, necrotizing enterocolitis (NEC), seizure, vomiting and pneumothorax was similar in the two groups.
Conclusion:
The results suggest that preventative caffeine can reduce the duration of NCPAP support in neonates with RDS.
Keywords: Apnea of prematurity, Caffeine, Distress, Preterm neonate, Respiratory Nasal continuous positive airway press
Copyright and License Information
© 2022 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Iranpour R, Armanian AM, Miladi N, Feizi A. Effect of prophylactic caffeine on noninvasive respiratory support in preterm neonates weighing 1250–2000 g: a randomized controlled trial. Arch Iran Med. 2022;25(2):98-104. doi: 10.34172/ aim.2022.16
Introduction
Methylxanthines have been used to prevent and decrease the rate of apnea in preterm neonates for many years.1 Among methylxanthines, caffeine is one of the most widely used prescription drugs because of its clinical efficacy, safety and minimal side effects and morbidity.2 The positive effects of caffeine are not limited to the prevention and treatment of apnea of prematurity (AOP).3
Studies have revealed additional significant benefits of caffeine on reducing the incidence of other neonatal complications, such as bronchopulmonary dysplasia,4 retinopathy of prematurity (ROP),5 intraventricular hemorrhage (IVH)6 and patent ductus arteriosus (PDA), especially in infants with birth weights less than 1250 g.7 Studies have addressed that prophylactic caffeine therapy reduces neurodevelopmental disorder at the ages of 18 and 24 months in extremely preterm neonates.8,9 Studies have shown the beneficial effect and safety of caffeine in earlier extubation and decreased the severity of chronic lung disease (CLD) in very low birth weight neonates.10,11 Currently, prophylactic caffeine is usually prescribed in the neonatal intensive care unit (NICU) for preterm neonates with respiratory distress syndrome (RDS) weighing less than 1250 g.12 Although the effect of respiratory stimulant caffeine in the neonatal population has been well established, routine use of prophylactic caffeine for preterm infants with birth weights greater than 1250 g receiving noninvasive respiratory support is not common.12,13 Our trial evaluated the effectiveness of prophylactic caffeine in premature infants with RDS who were under respiratory support with nasal CPAP, and their birth weights were 1250–2000 g. We examined the effect of caffeine therapy in reducing the duration of noninvasive respiratory support and oxygen requirements in two caffeine-treated and control groups. We also compared other factors, such as AOP, bronchopulmonary dysplasia, PDA, NEC, pneumothorax, IVH, seizure, nosocomial sepsis, ROP, and duration of hospitalization in the NICU, between the two groups.
Materials and Methods
Design and Subjects of the study
The present experiment was a prospective randomized controlled trial performed in the NICU of two distinct hospitals (Beheshti and Alzahra) both affiliated to Isfahan University of Medical Sciences, Iran, between December 2019 and June 2020.
The inclusion criteria were premature neonates with a gestation age of less than 37 weeks and birth weight between 1250 and 2000 g; weight appropriate for age; spontaneous respiration in neonates as well as clinical signs of respiratory distress requiring nasal CPAP. The exclusion criteria included sepsis in the first 3 days of birth; cyanotic cardiac disease; complex congenital anomalies; restriction of intrauterine growth; requirement for intubation and mechanically assisted ventilation in the initial 24 hours of life; and neonatal asphyxia (Apgar score of 0–3 at min 5, metabolic acidosis with umbilical cord blood gas analysis of pH <7 and base deficit <-12 mEq/L).
Intervention
The selected infants were randomly allocated in two groups: the caffeine (intervention) group, who had received intravenous caffeine citrate, and the control group, who had not received any placebo or similar drugs. Therefore, this study was not blinded. The initial loading dose of caffeine prescribed in the intervention group was 20 mg/kg. The researchers randomized the participants using random numbers generated by a computer in an algorithmic process. Allocation to treatment was not revealed until the study began.
Identical ventilator support protocols were used for the treatment of the neonates in both groups. Nasal continuous positive airway pressure (NCPAP) was initiated at a pressure of 6 cm of water. We used binasal midline prongs, supplied by Fisher & Paykel Healthcare (New Zealand). NCPAP was performed using a mechanical ventilator (Fabian, Acutronic Medical Systems; Zurich, Switzerland). In both groups, the levels of FIO2 were adjusted to keep the patients’ O2 saturation at 89%–95%.
Surfactant (Survanta; AbbVie Inc, Chicago, USA or Curosurf; Chiesa Pharmaceuticals, Parma, Italy) was prescribed if the neonates had FiO2 levels >30% to keep SPO2 at the desired levels. Surfactant was administered in accordance with the INSURE procedure (intubation-surfactant administration-extubation). In both groups, when the FiO2 level fell below 30% and respiratory distress improved, the patients were weaned to free oxygen in an incubator or an oxyhood; furthermore, when FiO2 levels reached 21%, oxygenation was terminated. NCPAP failure was considered in case of apnea or pH less than 7.2 and PaCO2 more than 60 mm Hg.
In the caffeine group, a daily dose of 10 mg/kg of caffeine was used as the daily maintenance dose until the infants tolerated without respiratory support with NCPAP or free oxygen administration. Maintenance doses were prescribed orally when enteral feeding was completed and the infants did not need intravenous fluids. Serum caffeine levels were not measured in this trial. Caffeine was discontinued if symptoms in favor of caffeine poisoning occurred, such as persistent tachycardia, unexplained seizures and significant vomiting.
Outcomes
The duration of respiratory support with NCPAP was considered the primary outcome. AOP, duration of need for oxygen, need for endotracheal intubation and mechanical ventilator, duration of NICU admission, diagnosis of PDA, unexplained seizure, necrotizing enterocolitis (NEC), nosocomial infection, air leak such as pneumothorax, IVH, ROP, CLD, vomiting, tachycardia, pulmonary hemorrhage and time required to achieve complete enteral feeding were regarded as secondary outcomes.
According to the definition, infants with CLD were considered when the need for supplemental oxygen persisted after the 28th day of life.14,15 The diagnostic criteria for NEC were intramural bowel gas (pneumatosis intestinalis), hepatobiliary gas, or free intraperitoneal air on radiography or the need for related surgical measures. IVH was detected using brain ultrasound. Cranial ultrasonography was recommended between the 3rd and 7th days of life by a trained neonatologist. The definition of IVH was based on the Papile classification.16 PDA was established by echocardiography. Tachycardia was defined as sinus rate exceeding 160-180 beats/min.17 The definition of AOP was considered by the cessation of breathing for more than 20 seconds and associated by hypoxia or bradycardia.18 ROP was recorded by an ophthalmologist according to the international classification.19 Late-onset sepsis was diagnosed with positive blood culture after 3 days of age.20
Everyday, a fellowship of neonatology closely monitored the primary and secondary outcomes. The data were entered in distinct forms after extraction from the NICU record sheets.
Statistical Analysis
The sample size, was calculated based on the formula: N = (φ+1/φ) (Z1-α/2 + Z1-β)2/Δ2 + (Z1-α/2)2/4. To detect a standardized effect size Δ = 0.6
21
based on the difference in the average time nasal CPAP as primary outcome in this study between the intervention and control groups, considering type one error rate α = 0.05, statistical power 1-β = 0.8 and attrition rate 20%, and equal number of patients in the intervention and control groups (φ = 1), the sample size needed for the current study was determined to be 45 patients in each group.
Continuous variables are presented as mean and standard deviation (SD), and categorical data are shown as frequencies (percentages). Normality of continuous variables was evaluated using Kolmogorov-Smirnov test and Q-Q plot, and non-normality positive skewed data were subjected to logarithmic transformation. The chi-square or Fisher exact tests were used to compare the proportions between the intervention and control groups. Independent samples t test or Mann-Whitney U test were used to compare the normally and non-normally distributed continuous data, respectively. The statically analyses were performed using SPSS version 20 (IBM, Armonk, NY, USA). All P values are two-sided and P < 0.05 was considered as statistically significant.
Results
Throughout the trial, overall 182 preterm newborns with birth weight ranging from 1250 to 2000 g underwent assessment for study eligibility. Totally, 92 infants were excluded for different reasons (Figure 1). Ninety-five subjects were randomly allocated into two groups, and 90 neonates completed the study (45 infants in each group). The demographic variables of the neonates were comparable for both groups at birth (Table 1). Baseline prenatal steroid use, steroid type (dexamethasone or betamethasone), number, age of surfactant administration and type of surfactant were not significantly different between the caffeine and control groups (Table 2).
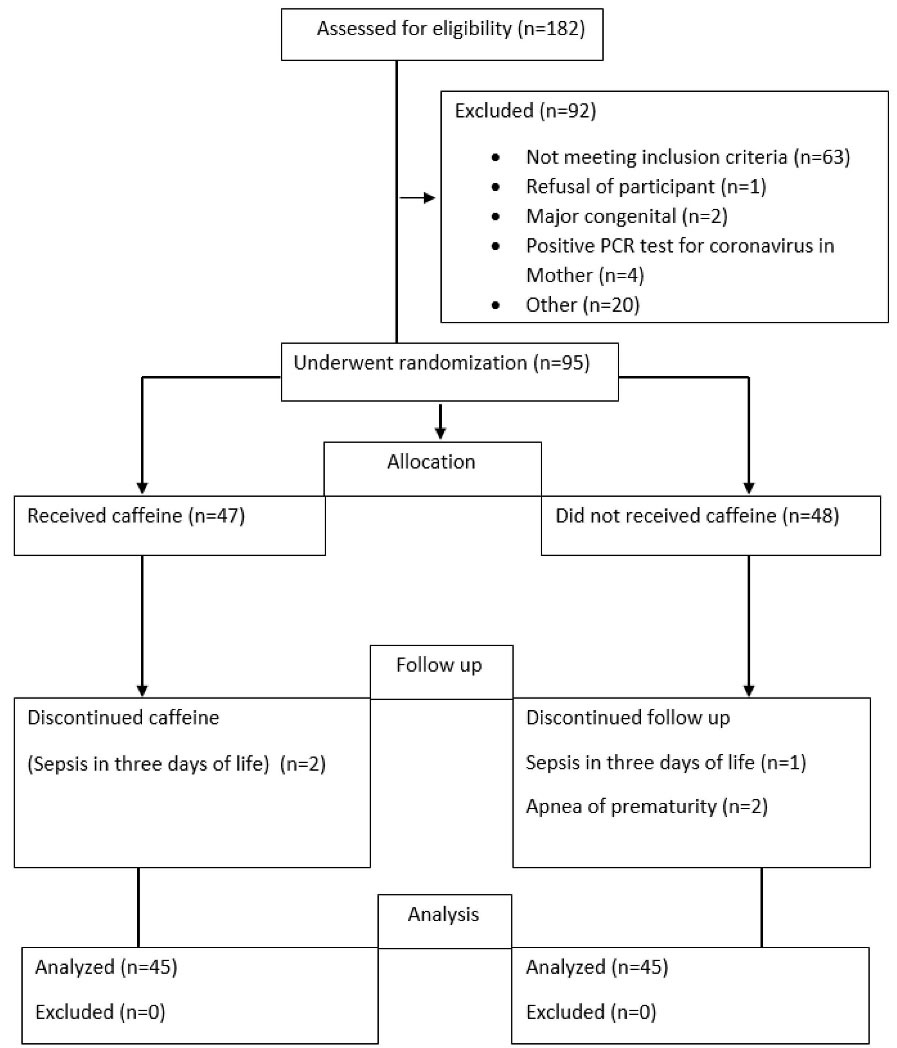
Figure 1.
Consort Flowchart of the Study.
.
Consort Flowchart of the Study.
Table 1.
Demographic and Basic Clinical Characteristics of Neonates in the Two Study Groups
|
Variables
|
Caffeine Group
(n = 45)
|
Control Group
(n = 45)
|
P
Value
|
| Gender |
Female* |
17 (37.8%) |
16 (35.6%) |
0.82a |
| Male* |
28 (62.2%) |
29 (64.4%) |
| Method of delivery |
Caesarian* |
40 (88.9%) |
39 (86.7%) |
0.74a |
| Vaginal delivery* |
5 (11.1%) |
6 (13.3%) |
| Gestational age, weeks** |
31.37 (1.55) |
31.50 (1.50) |
0.70 |
| Weight, g** |
1573.22 (220.99) |
1624.44 (223.29) |
0.27 |
| Height, cm** |
41.64 (2.86) |
42.34 (2.72) |
0.23 |
| Head circumference, cm** |
29.22 (1.18) |
28.98 (4.57) |
0.74 |
| Apgar score, first minute** |
6.17 (1.84) |
6.15 (2.28) |
0.96 |
| Apgar score, fifth minute** |
8.35 (1.28) |
8.22 (1.56) |
0.65 |
*Frequency (percentage), ** Mean (SD).
a Results from chi-square or Fisher exact tests and others were obtained from independent samples ttest
Table 2.
Prenatal Steroid Therapy and Surfactant Status of Neonates in the Two Groups
|
Variables
|
Caffeine Group
(n = 45)
|
Control Group
(n = 45)
|
P
Value
|
| Received prenatal steroids* |
34 (75.6%) |
26(57.8%) |
0.07 |
| Type of steroid* |
Dexamethasone |
2(4.4%) |
2(4.4%) |
0.19 |
| Betamethasone |
32(71.1%) |
24(53.3%) |
| Received surfactant* |
26 (57.8%) |
23 (51.1%) |
0.52 |
| Type of surfactant* |
Curusurf |
14(31.1%) |
13(28.9%) |
0.80 |
| Survanta |
12(26.7%) |
10(22.2%) |
| Age when receiving surfactant, hour** |
5.42 (6.15) |
6.78(8.53) |
0.52¥ |
| Number of surfactant received* |
Once |
21 (80.8%) |
12 (52.2%) |
0.17 |
| Twice |
3 (11.5%) |
6 (26.1%) |
| Three times |
2(7.7%) |
4(17.4%) |
| Four times |
0 |
1(4.3%) |
*Frequency (percentage), ** Mean (SD).
a Resulted from non-parametric Mann-Whitney U test and other P values were obtained from chi-square or Fisher exact tests.
Outcome Assessment
Outcomes in the two groups are shown in Table 3.The mean duration of NCPAP requirement, that was considered as the primary outcome, was statistically shorter in neonates who received caffeine than the neonates of the control group (P = 0.04)
IVH had a greater frequency among infants in the control group, compared to those in the caffeine group at 7 days after birth, showing a significant difference (P = 0.03), but during the first 72 hours after birth, the frequency of IVH was not significantly different between the two groups (P = 0.4; Table 3).
Table 3.
Primary and Secondary Outcomes in the Two Study Groups
|
Outcomes
|
Caffeine Group
(n = 45)
|
Control Group
(n = 45)
|
Mean/Proportion Difference (95% CI)
|
P
Value
|
| Duration requires NCPAP, hours (primary outcome)** |
41.53(43.25) |
78.48 (114.25) |
-36.9 (-73.14, -0.76) |
0.04b |
| Requires intubation and mechanical ventilator (NCPAP failure)* |
0 |
3 (6.7) |
-6.7% (-17.9, 2.31) |
0.07 |
| Need to free O2 after discontinuation of CPAP* |
43 (95.6) |
43 (95.6) |
- |
1 |
| Duration of free O2 need after discontinuation of NCPAP, hours |
157.84 (275.84) |
224.97 (298.36) |
-67.1 (-187.5, 53.2) |
0.27b |
Intraventricular Hemorrhage,
72 hours after Birth* |
Normal |
38 (84.4) |
36 (80) |
4.4% (-11.7, 2.3) |
0.43 |
| Grade 1 |
4 (8.9) |
2 (4.4) |
4.5% (-17.2, 16.8) |
| Grade 2 |
3 (6.7) |
6 (13.3) |
-6.6 (-20.2, 6.6) |
| Grade 3 |
0 |
1(2.2) |
-2.2% (-11.5, 5.9) |
Intraventricular Hemorrhage,
7days after birth* |
Normal |
40 (88.9) |
34 (75.6) |
13.3% (-2.7, 28.8) |
0.03 |
| Grade 1 |
5 (11.1) |
5 (11.1) |
- |
| Grade 2 |
0 |
6 (13.3) |
-13.3% (-26.1, -273) |
| Grade 3 |
- |
- |
- |
| AOP* |
2(4.4) |
9 (20) |
-15.6% (-29.8, -1.8) |
0.02 |
| Tachycardia* |
2(2.2) |
3 (6.7) |
-4.5% (-15.9, 5.8) |
0.64 |
| PDA* |
5 (11.1) |
9 (20) |
-8.9% (-24.1, 6.5) |
0.24 |
| CLD* |
9 (20) |
8 (17.8) |
2.2% (-18.4, 14.1) |
0.23 |
| NEC* |
0 |
1 (2.2) |
-2.2% (-11.5, 5.9) |
0.31 |
| ROP* |
1(2.2) |
2 (4.4) |
-2.2 (-12.7,7.7) |
0.55 |
| Pulmonary hemorrhage* |
0 |
0 |
- |
- |
| Pneumothorax* |
0 |
2 (4.4) |
-4.4% (-14.8, 4.1) |
0.15 |
| seizure |
0 |
1 (2.2) |
-2.2% (-11.5, 5.9) |
0.31 |
| Nosocomial infection* |
3(6.7) |
4 (8.9) |
-2.2% (-14.8,10.2) |
0.69 |
| Age when oral feeding began, hours** |
31.31 (22.81) |
36.4 (26.64) |
-5.1 (-15.5, 5.3) |
0.33b |
| Duration of full oral feeding, hours** |
153.86 (53.28) |
171.2 (68.45) |
-17.3 (-43.0, 8.4) |
0.18b |
| Time of hospitalization in NICU, day** |
9.22 (7.77) |
13.68 (11.84) |
-4.5 (-8.7, -0.3) |
0.04 a |
| Number of vomiting during study* |
None |
28 (62.2) |
29 (64.4) |
-2.2% (-21.3, 17.1) |
0.47 |
| Once |
8 (17.8) |
4 (8.9) |
8.9% (-5.5, 23.5) |
| Twice |
5 (11.1) |
4 (8.9) |
2.2% (-11.2, 15.7%) |
| Three times |
4 (8.9) |
5 (11.1) |
-2.2% (-15.7%, 11.2) |
| Four times |
0 |
1 (2.2) |
-2.2% (-11.5, 5.9) |
| Five times |
0 |
2 (4.4) |
-4.4% (-14.8, 4.1) |
NCPAP, nasal continuous positive airway pressure; AOP, apnea of prematurity; PDA, patent ductus arteriosus; CLD, Chronic lung disease; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity;
*Frequency (percentage), ** Mean (SD),
a Obtained based on Mann-Whitney test, b Resulted from independent samples t test and other P values were obtained by chi-square or Fisher exact tests.
The mean duration of hospitalization in the NICU was statistically longer in the control group than the caffeine group (P = 0.04; Table 3). The incidence of apnea of prematurity (AOP) differed between the two groups. Apnea was observed in only two newborns in the caffeine group, in comparison with nine (20%) in the control group (P = 0.02).
Other secondary outcomes, such as NCPAP failure (need for intubation and ventilator), incidence of PDA, pneumothorax, ROP, seizure, vomiting, nosocomial infection, pulmonary hemorrhage, CLD, and enterocolitis were not significantly different in the intervention and control groups (Table 3). Additionally, there were no deaths in either group.
Discussion
In our randomized clinical trial, caffeine could reduce the mean duration required for NCPAP in infants with RDS. Furthermore, the mean duration of hospitalization in the NICU, rate of IVH and incidence of AOP in the caffeine group were clearly reduced compared to those in the control group. Concerning the other secondary outcomes, the researchers did not find any differences of significance between the caffeine and the control groups (Table 3). Studies have reported the incidences of CPAP failure requiring “rescue” to range from 22% to 36% for VLBW infants22 and 46% to 66% for extremely preterm infants.23 Multiple studies have shown that early caffeine administration is effective and safe to decrease both the incidence of apnea of prematurity and the use of mechanical ventilation in premature infants with RDS.24,25 In the CAP trial (Caffeine for Apnea of Prematurity trial), infants weighing 500 to1250 g were divided into caffeine and placebo groups in the first 10 days of life.26 In this randomized trial, caffeine-treated infants were separated from mechanical ventilator and oxygen therapy one week earlier than infants receiving placebo. In addition, this trial showed that as prophylaxis for AOP, caffeine can reduce the incidence of BPD. Currently, caffeine is widely used as a prophylactic drug for apnea in preterm infants with RDS and birth weights less than 1250 g.12 However, few studies have evaluated whether prescription of prophylactic caffeine from the first day of birth can increase the success rate of noninvasive treatment (such as NCPAP), particularly in VLBW newborns with birth weights greater than 1250 g. Our study supports the hypothesis that early caffeine therapy has beneficial effects, even in premature neonates with a birth weight more than 1250 g. However, our study did not show a decreased incidence of NCPAP failure or BPD. This study revealed that early caffeine therapy reduces the incidence of AOP and the duration of noninvasive respiratory support. The reason why our study did not demonstrate the effectiveness of caffeine in reducing BPD may be because we selected infants with birth weights greater than 1250 g.
Furthermore, studies have demonstrated that caffeine has other benefits, such as reduced need for PDA treatment, decrease in severity of ROP and a significant decrease in the incidence of intracranial hemorrhage.26-28 Our trial did not show that caffeine prophylaxis was associated with a reduction in the incidence of ROP and PDA but could decrease the incidence of IVH at 7 days after birth. We know that the incidence of PDA and ROP is inversely related to weight and gestational age. Our neonates in the caffeine and control groups were not extremely premature. We assumed that the incidence of BPD and ROP was low in both groups. Therefore, the difference between the two groups may not be significant. However, it is also possible that if we had a larger sample size, the difference would have been significant similar to IVH. Caffeine has various dose-related adverse effects on different organs. Methylxanthines may delay the emptying of stomach content and reduce the tonicity of the lower esophageal sphincter; therefore, they may increase gastroesophageal reflux in premature neonates.29 Clinical trials have not reported an increased incidence of gastroesophageal reflux in caffeine-treated preterm infants.30 In the current study, caffeine citrate administration did not aggravate vomiting. Furthermore, our trial showed that caffeine did not increase the time required to complete oral feeding compared to the no-caffeine group. Studies have demonstrated that caffeine administration in infants is not associated with an increased risk of NEC compared to the control group.31 Our study also showed that caffeine did not aggravate the incidence of NEC. Caffeine intoxication in preterm infants is associated with tachycardia, tachypnea, tremor, and seizure activity.32 However, studies have shown that caffeine citrate with an initial dose of 20 mg/kg and then a daily maintenance dose of 5–10 mg/kg does not cause significant side effects such as tachycardia and seizure.26,27 In the present study, the researchers did not find any significant increments in the incidence of tachycardia or seizures in the caffeine group. These results suggest that early prophylactic caffeine therapy is safe to reduce the duration of respiratory therapy with NCPAP in infants with respiratory distress even in premature neonates with birth weights more than 1250 g.
Our study had some limitations. First, due to some ethical limitations, placebo was not used in the control group; therefore, our trial was not double-blinded. Therefore, there is a possibility of bias of evaluation in our study. Second, although the primary outcome was significantly different between the intervention and control groups, caution should be taken in interpreting these results. Our sample size was small, and for routine use of caffeine as part of a non-invasive respiratory treatment in all premature newborns diagnosed with RDS, further trials, particularly multicenter research, are needed.
In conclusion, this randomized controlled trial concluded that initial prophylactic caffeine therapy in premature neonates with birth weights of 1250–2000 g is effective in decreasing the duration of noninvasive support, AOP and hospitalization. In addition, this study did not reveal any significant adverse effects with routine caffeine doses. However, to be able to recommend the widespread use of caffeine as a prophylactic treatment in managing all preterm neonates with RDS, there is need for further research.
Acknowledgements
The researchers would like to sincerely thank the parents of the patients who participated in this trial.
Authors’ Contribution
RI, AF and AMA have designed the study and established final approval. NM collected and managed the data. AF performed statistical analysis. RI, NM and AMA prepared the manuscript. RI and AF made revisions of the manuscript. The authors read and accepted with the final manuscript.
Conflict of Interest Disclosures
None declared.
Ethical Statement
The protocol of the study was approval by the ethics committee of the Isfahan University of Medical Sciences (Ethics committee Ref. No.: IR.MUI.MED.REC.1398.355). This study has been registered at IRCT.ir (identifier: IRCT20170627034782N2). Registration date: 2019-12-12, 1398/09/21.
Funding
The present project was financed by the Isfahan University of Medical Sciences.
References
- Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK Jr, Smith PB. Medication use in the neonatal intensive care unit. Am J Perinatol 2014; 31(9):811-21. doi: 10.1055/s-0033-1361933 [Crossref] [ Google Scholar]
- Krzyżaniak N, Pawłowska I, Bajorek B. Review of drug utilization patterns in NICUs worldwide. J Clin Pharm Ther 2016; 41(6):612-20. doi: 10.1111/jcpt.12440 [Crossref] [ Google Scholar]
- Dobson NR, Patel RM. The role of caffeine in noninvasive respiratory support. Clin Perinatol 2016; 43(4):773-82. doi: 10.1016/j.clp.2016.07.011 [Crossref] [ Google Scholar]
- Taha D, Kirkby S, Nawab U, Dysart KC, Genen L, Greenspan JS. Early caffeine therapy for prevention of bronchopulmonary dysplasia in preterm infants. J Matern Fetal Neonatal Med 2014; 27(16):1698-702. doi: 10.3109/14767058.2014.885941 [Crossref] [ Google Scholar]
- Kua KP, Lee SW. Systematic review and meta-analysis of clinical outcomes of early caffeine therapy in preterm neonates. Br J Clin Pharmacol 2017; 83(1):180-91. doi: 10.1111/bcp.13089 [Crossref] [ Google Scholar]
- Helwich E, Rutkowska M, Bokiniec R, Gulczyńska E, Hożejowski R. Intraventricular hemorrhage in premature infants with respiratory distress syndrome treated with surfactant: incidence and risk factors in the prospective cohort study. Dev Period Med 2017; 21(4):328-35. doi: 10.34763/devperiodmed.20172104.328335 [Crossref] [ Google Scholar]
- Moschino L, Zivanovic S, Hartley C, Trevisanuto D, Baraldi E, Roehr CC. Caffeine in preterm infants: where are we in 2020?. ERJ Open Res 2020; 6(1):00330-2019. doi: 10.1183/23120541.00330-2019 [Crossref] [ Google Scholar]
- Schmidt B, Roberts RS, Anderson PJ, Asztalos EV, Costantini L, Davis PG. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: an 11-year follow-up of the CAP randomized clinical trial. JAMA Pediatr 2017; 171(6):564-72. doi: 10.1001/jamapediatrics.2017.0238 [Crossref] [ Google Scholar]
- Lodha A, Entz R, Synnes A, Creighton D, Yusuf K, Lapointe A. Early caffeine administration and neurodevelopmental outcomes in preterm infants. Pediatrics 2019; 143(1):e20181348. doi: 10.1542/peds.2018-1348 [Crossref] [ Google Scholar]
- Doyle LW, Ranganathan S, Cheong JLY. Neonatal caffeine treatment and respiratory function at 11 years in children under 1,251 g at birth. Am J Respir Crit Care Med 2017; 196(10):1318-24. doi: 10.1164/rccm.201704-0767OC [Crossref] [ Google Scholar]
- Dobson NR, Hunt CE. Caffeine: an evidence-based success story in VLBW pharmacotherapy. Pediatr Res 2018; 84(3):333-40. doi: 10.1038/s41390-018-0089-6 [Crossref] [ Google Scholar]
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology 2019; 115(4):432-50. doi: 10.1159/000499361 [Crossref] [ Google Scholar]
- Davis PG. When to start and stop caffeine and why respiratory status matters. Semin Fetal Neonatal Med 2020; 25(6):101175. doi: 10.1016/j.siny.2020.101175 [Crossref] [ Google Scholar]
- Goldsmith JP, Karotkin E. Assisted Ventilation of the Neonate. 6th ed. Philadelphia: Elsevier; 2017.
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163(7):1723-9. doi: 10.1164/ajrccm.163.7.2011060 [Crossref] [ Google Scholar]
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978; 92(4):529-34. doi: 10.1016/s0022-3476(78)80282-0 [Crossref] [ Google Scholar]
- Martin RJ, Fanaroff AA, Walsh MC. Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. Philadelphia: Elsevier; 2014.
- Barrington K, Finer N. The natural history of the appearance of apnea of prematurity. Pediatr Res 1991; 29(4 Pt 1):372-5. doi: 10.1038/pr.1991.72500 [Crossref] [ Google Scholar]
- Patz A. Patz AAn international classification of retinopathy of prematurityIIThe classification of retinal detachment. Arch Ophthalmol 1987; 105(7):905. doi: 10.1001/archopht.1987.01060070041024 [Crossref] [ Google Scholar]
- Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr 1996; 129(1):63-71. doi: 10.1016/s0022-3476(96)70191-9 [Crossref] [ Google Scholar]
- Armanian AM, Iranpour R, Faghihian E, Salehimehr N. Caffeine administration to prevent apnea in very premature infants. Pediatr Neonatol 2016; 57(5):408-12. doi: 10.1016/j.pedneo.2015.10.007 [Crossref] [ Google Scholar]
- Ammari A, Suri M, Milisavljevic V, Sahni R, Bateman D, Sanocka U. Variables associated with the early failure of nasal CPAP in very low birth weight infants. J Pediatr 2005; 147(3):341-7. doi: 10.1016/j.jpeds.2005.04.062 [Crossref] [ Google Scholar]
- Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 2010; 362(21):1970-9. doi: 10.1056/NEJMoa0911783 [Crossref] [ Google Scholar]
- Erenberg A, Leff RD, Haack DG, Mosdell KW, Hicks GM, Wynne BA. Caffeine citrate for the treatment of apnea of prematurity: a double-blind, placebo-controlled study. Pharmacotherapy 2000; 20(6):644-52. doi: 10.1592/phco.20.7.644.35167 [Crossref] [ Google Scholar]
- Henderson-Smart DJ, De Paoli AG. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst Rev 2010(12):CD000140. doi: 10.1002/14651858.CD000140.pub2 [Crossref]
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A. Caffeine therapy for apnea of prematurity. N Engl J Med 2006; 354(20):2112-21. doi: 10.1056/NEJMoa054065 [Crossref] [ Google Scholar]
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 2007; 357(19):1893-902. doi: 10.1056/NEJMoa073679 [Crossref] [ Google Scholar]
- Park HW, Lim G, Chung SH, Chung S, Kim KS, Kim SN. Early caffeine use in very low birth weight infants and neonatal outcomes: a systematic review and meta-analysis. J Korean Med Sci 2015; 30(12):1828-35. doi: 10.3346/jkms.2015.30.12.1828 [Crossref] [ Google Scholar]
- Gounaris A, Kokori P, Varchalama L, Konstandinidi K, Skouroliakou M, Alexiou N. Theophylline and gastric emptying in very low birthweight neonates: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2004; 89(4):F297-9. doi: 10.1136/adc.2003.027565 [Crossref] [ Google Scholar]
- Mezzacappa MA, Rosa AC. Clinical predictors of abnormal esophageal pH monitoring in preterm infants. Arq Gastroenterol 2008; 45(3):234-8. doi: 10.1590/s0004-28032008000300013 [Crossref] [ Google Scholar]
- Lampkin SJ, Turner AM, Lakshminrusimha S, Mathew B, Brown J, Fominaya CE. Association between caffeine citrate exposure and necrotizing enterocolitis in preterm infants. Am J Health Syst Pharm 2013; 70(7):603-8. doi: 10.2146/ajhp120457 [Crossref] [ Google Scholar]
- Ergenekon E, Dalgiç N, Aksoy E, Koç E, Atalay Y. Caffeine intoxication in a premature neonate. Paediatr Anaesth 2001; 11(6):737-9. doi: 10.1046/j.1460-9592.2001.00753.x [Crossref] [ Google Scholar]