Arch Iran Med. 24(9):713-721.
doi: 10.34172/aim.2021.104
Systematic Review
Maternal and Neonatal Complications, Outcomes and Possibility of Vertical Transmission in Iranian Women with COVID-19
Zohreh Heidary 1  , Omid Kohandel 2, Hanieh Fathi 2, Majid Zaki-Dizaji 3, Marjan Ghaemi 1, Batool Hossein Rashidi 1, *
, Omid Kohandel 2, Hanieh Fathi 2, Majid Zaki-Dizaji 3, Marjan Ghaemi 1, Batool Hossein Rashidi 1, * 
Author information:
1Vali-e-Asr Reproductive Health Research Center, Tehran University of Medical Sciences, Tehran, Iran
2Student Research Committee of Alborz University of Medical Sciences, Karaj, Iran
3Legal Medicine Research Center, Legal Medicine Organization, Tehran, Iran
*Corresponding Author: Batool Hossein Rashidi, MD; Vali-e-Asr Reproductive Health Research Center, Tehran University of Medical Sciences, Tehran, Iran. Tel: +98-21-66581616; Email:
bhrashidi@gmail.com
Abstract
Background:
The emergence and fast spread of coronavirus disease 2019 (COVID-19) threatens the world as a new public health crisis. Little is known about its effects during pregnancy. This study aimed to investigate the clinical manifestations of COVID-19 on maternal and neonatal outcomes.
Methods:
In this systematic review, PubMed, Scopus, Web of Science, and Google Scholar databases were searched focusing on pregnancy and perinatal outcomes of COVID-19.
Results:
The initial search yielded 1236 articles, from which finally 21 unique studies, involving 151 pregnant women and 17 neonates, met the criteria. Mean ± SD age of included mothers and mean ± SD gestational age at admission were 30.6 ± 6.2 years and 30.8 ± 8.9 weeks, respectively. The common symptoms were fever, cough, fatigue, dyspnea and myalgia. The mortality rates of pregnant women and neonates were 28 out of 151 (18.5%) and 4 out of 17 (23.5%), respectively. Most of the neonates were preterm at the time of delivery. Three neonates had positive RT-PCR test on the first day after birth and three others on day two. On the average, neonate’s PCR became positive on day 4 for the first time.
Conclusion:
Early diagnosis of COVID-19 is crucial due to the possibility of the prenatal complications. Strict prevention strategies may reduce the risk of mother to infant transmission.
Keywords: COVID-19, Pregnancy, SARS-CoV-2, Systematic Review, Vertical Transmission
Copyright and License Information
© 2021 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Heidary Z, Kohandel Gargari O, Fathi H, Zaki-Dizaji M, Ghaemi M, Hossein Rashidi B. Maternal and neonatal complications, outcomes and possibility of vertical transmission in iranian women with COVID-19. Arch Iran Med. 2021;24(9): 713-721. doi: 10.34172/aim.2021.104
Introduction
The emergence and fast spread of novel coronavirus disease 2019 (COVID-19) which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), threatens the world as a new public health crisis. As of January 16, 2021, there were nearly 94 million confirmed cases of SARS-CoV-2 infection with more than 2 million deaths worldwide according to the World Health Organization (WHO).1 These numbers are increasing daily and crude mortality is estimated at 0.8–10.2% for the twenty countries currently most affected by COVID-19.2
It is critical to identify at-risk and vulnerable individuals for COVID-19 to avoid adverse outcomes with early preventive management. Although coronavirus can infect anyone, pregnant women are more susceptible to this virus due to physiological and immunological alterations during pregnancy.3,4 Currently, there is very limited knowledge about the various aspects of the coronavirus including during pregnancy and maternal and neonatal health before and after delivery. National-level as well as global studies are now required to provide reliable data on the incidence of COVID-19 in pregnancy and its effects on the pregnant woman, fetus, and newborn.
The objective of the present systematic review was to evaluate the clinical characteristics of COVID-19 and maternal, fetal, and neonatal outcomes published in the literature in the Iranian population and answer the following issues: (i) describe the clinical characteristics of COVID-19 in pregnant women; (ii) discuss obstetric outcomes; (iii) describe the risk of vertical transmission; (iv) use this data to inform clinical practice and guide policy in maternity services; and (v) identify gaps in the existing knowledge.
Materials and Methods
Search Strategy
The present systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines.5 PubMed, Scopus, Web of Science, and Google scholar databases were searched for studies on pregnant women and neonates with COVID-19 infection from December 1, 2019 until December 31, 2020.
For the search strategy, combinations of the following keywords and medical subject heading (MeSH) terms were used: “coronavirus OR COVID-19 OR COVID 19 OR SARS-CoV-2 OR n19-CoV”, “newborn OR neonatal OR child OR infants”, “pregnancy OR pregnant OR maternal OR mother”, “vertical transmission OR maternal–fetal transmission OR intrauterine transmission”. The above three combinations were searched alone or together, with also “Iran OR Iranian” keywords.
Study Registration
The protocol of this study review was registered with Pajooheshyar under registration number 93-3-94-50455. This study is classified as a systematic review and no institutional review board approval was required.
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (1) Only observational studies including case reports, case series and cohort studies were found eligible. (2) Participants must be pregnant with confirmed SARS-CoV-2 infection by RT-PCR or CT scan. (3) Study must take place in Iranian hospitals. (4) Neonates must be born to mothers with confirmed SARS-CoV-2 infection. (5) Only newborns who were not discharged after delivery were found eligible. (6) Skin to skin contact must be limited after birth and during breast feeding.
Exclusion criteria were as follows: review articles, opinion pieces or guidelines, articles pertaining solely to MERS-CoV, SARS-CoV or other viruses, non-peer-reviewed papers, unpublished reports, articles in which the date and location of the study were not specified, women with suspected COVID-19 which was not confirmed by laboratory tests.
Study Selection
Titles and abstracts were independently retrieved and reviewed for eligibility by two authors (H.F. and Z.H.) and non-relevant studies and studies that did not meet the inclusion criteria were excluded. After the initial screening, the remaining full texts were reviewed by O.K.G, and articles with insufficient data and duplicates were identified. Eligibility of the included studies and cases reported in case series were double checked at this stage. Then, all studies were divided into 3 major groups: maternal outcome, neonatal outcome and vertical transmission possibility.
Data Extraction, Quality Assessment and Outcome Measures
Two authors (Z.H. and H.F.) extracted the required information from selected studies. The extracted information included first author’s name, study type, number of participants for case series and cohort studies, participant’s age, gestational age, clinical symptoms of mothers and newborns, laboratory finding of mothers and newborns, PCR tests results, imaging findings of both mothers and newborns, delivery type and protection strategies.
The methodological quality of the studies was assessed independently by the same two authors using the Joanna Briggs Institute (JBI) tool for case series and case reports.6
Data Analysis
Due to lack of data, we decided to perform a narrative systematic review without meta-analysis (SWiM). Central and descriptive statistics were reported for quantitative data. All statistical analyses were performed using the SPSS software (v. 26.0, Chicago, IL). We used a method presented by Hozo et al7 to estimate the median, average and standard deviation of some variables in cohort studies like WBC count.8
Results
Study Selection and Characteristics
The initial search yielded 1236 articles. Thirty-seven articles were duplicates, and 1133 articles were excluded following title and abstract screening. A total of 34 articles were removed due to lack of relevancy and 8 articles were removed because the maternal PCR was not positive or the newborns were discharged after delivery. Finally, 21 unique studies involving 168 participants met the criteria for full review (Figure 1).
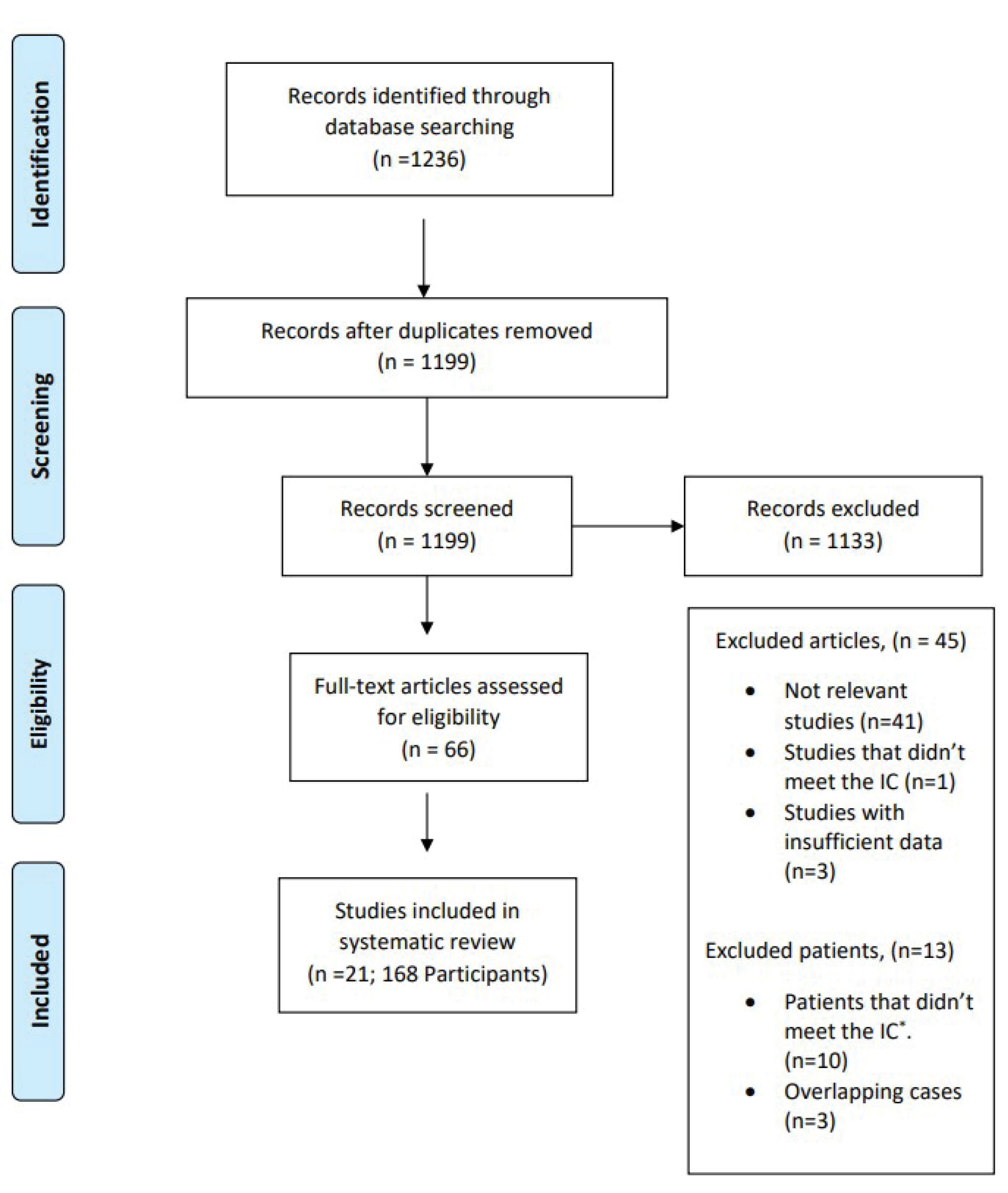
Figure 1.
PRISMA Flowchart Showing Selection of Studies For review. IC, Inclusion criteria.
.
PRISMA Flowchart Showing Selection of Studies For review. IC, Inclusion criteria.
Additionally, the study by Dorgalale et al9 was found eligible because the neonate returned to hospital with symptoms on the day he was discharged. In another case series by Schwartz et al,10 10 cases were excluded because the mothers’ RT-PCR was negative or not reported and one case was excluded because the neonate was discharged after delivery. The triplets reported by Farsi et al11 were overlapping with one of the cases in the Schwartz et al10 case series.
Maternal Clinical Characteristics and Outcomes
To identify COVID-19 complications among Iranian pregnant women, 12 observational studies including 7 case reports, 2 case series and 3 cohort studies were selected. Totally, 151 pregnant women were included in this study. The characteristics of these articles are shown in Table 1. The mean ± SD age of included mothers and gestational age at admission were 30.56 ± 6.28 years and 30.82 ± 8.9 weeks, respectively. Gestational age was not reported in one of the cohort studies and two other cohort studies only mentioned average gestational age for all of their participants. Out of 20 pregnant women, one was in the first trimester (5%), 4 were in their second trimester (20%) and the other 15 were in the third trimester (75%). The mortality rate in pregnant women was 28 out of 151 (18.5%). Only 16 patients were reported to be admitted to the Intensive Care Unit (ICU) but unfortunately, ICU admission was not reported in most cases and the exact number is bigger than 16. Due to this fact, we did not consider this variable in our study.
Table 1.
Characteristics of Included Studies to Evaluate the Maternal Outcome of COVID-19
|
Study Type
|
First Author (Ref)
|
Number of Participants (n)
|
MA
(y)
|
Maternal Mortality (Count)
|
ICU Admission (Count)
|
Ventilator Support (Count)
|
GA at Admission (wk)
|
Delivery
|
|
C/S
|
NVD
|
Still Pregnant
|
| Case reports |
Jafari12 |
1 |
26.00 |
0 |
N/R |
N/R |
36.00 |
1 |
0 |
0 |
| Karami13 |
1 |
27.00 |
1 |
1 |
0 |
34.00 |
0 |
1 |
0 |
| Khodamoradi14 |
1 |
36.00 |
0 |
N/R |
N/R |
37.00 |
1 |
0 |
0 |
| Mohammadi15 |
1 |
26.00 |
0 |
N/R |
N/R |
8.00 |
0 |
0 |
0 |
| Soleimani16 |
1 |
30.00 |
0 |
1 |
0 |
21.20 |
0 |
0 |
0 |
| Taghizadieh17 |
1 |
33.00 |
0 |
N/R |
1 |
34.00 |
1 |
0 |
0 |
| Zamaniyan18 |
1 |
22.00 |
1 |
1 |
1 |
32.00 |
1 |
0 |
0 |
| Total |
7 |
28.57 |
2 |
3 |
2 |
28.89 |
4 |
1 |
0 |
| Case series |
Gheysarzadeh19 |
| Case1 |
1 |
30.00 |
0 |
N/R |
0 |
40.00 |
0 |
1 |
0 |
| Case2 |
1 |
42.00 |
0 |
N/R |
0 |
40.00 |
1 |
0 |
0 |
| Case3 |
1 |
30.00 |
0 |
N/R |
0 |
22.00 |
0 |
0 |
1 |
| Case4 |
1 |
27.00 |
0 |
N/R |
0 |
34.00 |
0 |
0 |
1 |
| Hantoushzadeh20 |
| Case1 |
1 |
39.00 |
1 |
1 |
1 |
36.00 |
1 |
0 |
0 |
| Case2 |
1 |
39.00 |
1 |
N/R |
1 |
33.80 |
1 |
0 |
0 |
| Case3 |
1 |
49.00 |
1 |
1 |
1 |
28.00 |
1 |
0 |
0 |
| Case4 |
1 |
39.00 |
1 |
N/R |
1 |
24.00 |
0 |
0 |
0 |
| Case5 |
1 |
34.00 |
1 |
1 |
1 |
36.00 |
1 |
0 |
0 |
| Case6 |
1 |
34.00 |
1 |
N/R |
1 |
24.00 |
0 |
0 |
0 |
| Case7 |
1 |
44.00 |
1 |
N/R |
1 |
30.80 |
1 |
0 |
0 |
| Case8 |
1 |
29.00 |
1 |
1 |
1 |
38.50 |
1 |
0 |
0 |
| Case9 |
1 |
29.00 |
1 |
1 |
1 |
30.50 |
0 |
0 |
0 |
| Total |
13 |
35.77 |
9 |
5 |
9 |
32.12 |
7 |
1 |
2 |
| Cohorts |
Amini Moghadam21 |
15 |
30.00 |
15 |
N/R |
N/R |
N/R |
0 |
0 |
0 |
| Pirjani8 |
66 |
30.90 |
0 |
4 |
N/R |
32.60 |
0 |
0 |
0 |
| Sattari22 |
50 |
29.20 |
2 |
4 |
N/R |
28.40 |
10 |
14 |
0 |
| Total |
131 |
30.15 |
17 |
8 |
N/R |
30.79 |
10 |
14 |
2 |
| Total |
|
151 |
30.56
(6.28) |
28
(18.5%) |
16
(10.5%) |
11
(7.28%) |
30.82
(8.9) |
21
(13.9%) |
16
(10.5%) |
2
(1.3%) |
GA, Gestational age; ICU, Intensive care unit; MA, Maternal age; C/S, Cesarean section; NVD, Normal vaginal delivery Totals are presented as number (percentage) or average (Standard deviation).
The most common symptoms among our participants were fever (n = 102, 67.5%), cough (n = 84, 55.6%), fatigue (n = 68, 45%), dyspnea (n = 65, 45%) and myalgia (n = 39, 25.8%). Gastrointestinal symptoms such as nausea (n = 14, 1%), vomiting (n = 14, 1%) and diarrhea (n = 7, 0.5%) were also present in some patients. All symptoms and clinical and laboratory data have been summarized in Table 2 and Table S1 (see Supplementary file 1
8,12-22
).
Table 2.
Laboratory and Examination Findings of Included Pregnant Mothers
|
Study type
|
First Author
|
Systolic
Pressure
|
Diastolic
Pressure
|
RR
|
HR
|
WBC (×10
9
/L)
|
Lymph
percentage
|
CRP
|
SpO2
|
BUN
|
Cr
|
ESR
|
AST
|
ALT
|
Plt
|
PT
|
PTT
|
D-dimer
|
| Case Reports |
Jafari12 |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
92.00 |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
| Karami13 |
100.00 |
60.00 |
37.00 |
110.00 |
10.00 |
7.20 |
31.00 |
93.00 |
N/R |
2.80 |
54.00 |
52.00 |
68.00 |
40.00 |
N/R |
N/R |
2.00 |
| Khodamoradi14 |
110.00 |
70.00 |
20.00 |
92.00 |
N/R |
N/R |
N/R |
94.00 |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
800.00 |
| Mohammadi15 |
96.00 |
74.00 |
27.00 |
N/R |
3.20 |
N/R |
N/R |
95.00 |
7.00 |
0.80 |
N/R |
123.00 |
283.00 |
359.00 |
N/R |
N/R |
N/R |
| Soleimani16 |
110.00 |
70.00 |
N/R |
98.00 |
N/R |
N/R |
N/R |
76.00 |
N/R |
10.00 |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
| Taghizadieh17 |
100.00 |
66.00 |
36.00 |
N/R |
11.00 |
N/R |
N/R |
94.00 |
N/R |
6.80 |
N/R |
78.00 |
46.00 |
N/R |
16.00 |
36.00 |
N/R |
| Zamaniyan18 |
N/R |
N/R |
26.00 |
118.00 |
7.80 |
* |
32.90 |
85.00 |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
| Case reports avg |
103.20 |
68.00 |
29.20 |
104.50 |
8.00 |
7.20 |
31.95 |
89.86 |
7.00 |
5.10 |
54.00 |
84.33 |
99.28 |
199.50 |
16.00 |
36.00 |
401.00 |
| Case Series |
Gheysarzadeh19 |
| Case1 |
115.00 |
75.00 |
18.00 |
95.00 |
N/R |
N/R |
2.00 |
97.00 |
N/R |
N/R |
13.00 |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
| Case2 |
110.00 |
70.00 |
20.00 |
90.00 |
7.70 |
18.00 |
3.00 |
96.00 |
N/R |
1.00 |
20.00 |
N/R |
N/R |
359.00 |
N/R |
N/R |
N/R |
| Case3 |
120.00 |
80.00 |
19.00 |
100.00 |
6.20 |
19.00 |
N/R |
94.00 |
N/R |
0.80 |
125.00 |
17.00 |
12.30 |
199.00 |
N/R |
N/R |
N/R |
| Case4 |
120.00 |
80.00 |
14.00 |
86.00 |
6.10 |
34.00 |
N/R |
N/R |
N/R |
0.60 |
N/R |
N/R |
N/R |
196.00 |
N/R |
N/R |
N/R |
| Hantoushzadeh20 |
| Case1 |
N/R |
N/R |
N/R |
N/R |
26.00 |
8.50 |
210.00 |
85.00 |
N/R |
0.60 |
N/R |
172.00 |
126.00 |
145.00 |
N/R |
N/R |
N/R |
| Case2 |
130.00 |
80.00 |
N/R |
N/R |
9.40 |
9.00 |
45.00 |
60.00 |
N/R |
0.60 |
N/R |
80.00 |
62.00 |
275.00 |
N/R |
N/R |
N/R |
| Case3 |
120.00 |
80.00 |
N/R |
N/R |
16.40 |
7.00 |
81.90 |
62.50 |
N/R |
0.70 |
N/R |
66.00 |
38.00 |
380.00 |
N/R |
N/R |
N/R |
| Case4 |
N/R |
N/R |
N/R |
N/R |
7.00 |
9.00 |
117.50 |
65.00 |
N/R |
0.90 |
N/R |
29.00 |
18.00 |
177.00 |
N/R |
N/R |
N/R |
| Case5 |
N/R |
N/R |
N/R |
N/R |
20.30 |
8.50 |
64.00 |
72.50 |
N/R |
0.80 |
N/R |
40.00 |
17.00 |
305.00 |
N/R |
N/R |
N/R |
| Case6 |
N/R |
N/R |
30.00 |
130.00 |
13.30 |
7.70 |
56.00 |
83.00 |
N/R |
6.00 |
N/R |
28.00 |
26.00 |
206.00 |
N/R |
N/R |
N/R |
| Case7 |
N/R |
N/R |
N/R |
N/R |
7.00 |
5.00 |
25.00 |
55.00 |
N/R |
0.60 |
N/R |
160.00 |
143.00 |
224.00 |
N/R |
N/R |
N/R |
| Case8 |
110.00 |
70.00 |
N/R |
N/R |
8.00 |
N/R |
18.00 |
70.00 |
N/R |
0.50 |
N/R |
60.00 |
40.00 |
68.00 |
N/R |
N/R |
N/R |
| Case9 |
125.00 |
80.00 |
N/R |
N/R. |
3.80 |
6.80 |
41.00 |
85.00 |
N/R |
0.80 |
N/R |
52.00 |
68.00 |
51.00 |
N/R |
N/R |
N/R |
| Case series avg |
118.75 |
76.88 |
20.20 |
100.20 |
10.93 |
12.05 |
60.31 |
77.08 |
N/R |
1.16 |
52.67 |
70.40 |
55.03 |
215.42 |
N/R |
N/R |
N/R |
| Cohorts |
Amini Moghadam21 |
N/R |
N/R |
N/R |
N/R |
12.10 |
* |
N/R |
78.00 |
N/R |
0.90 |
N/R |
27.00 |
30.00 |
171.00 |
N/R |
N/R |
N/R |
| Pirjani8 |
110.00** |
N/R |
18.00** |
94.00** |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R |
N/R. |
N/R |
N/R |
N/R |
N/R |
| Sattari22 |
N/R |
N/R |
N/R |
N/R |
10.60 |
19.50 |
1.84 |
91.60 |
N/R |
0.77 |
N/R |
N/R |
23.40 |
217.00 |
N/R |
N/R |
N/R |
| Cohorts avg |
110.00 |
N/R |
18.00 |
94.00 |
10.95 |
19.50 |
1.84 |
88.46 |
N/R |
0.80 |
N/R |
27.00 |
24.92 |
206.38 |
N/R |
N/R |
N/R |
| Total |
|
110.46
(12.86) |
73.46
(6.46) |
18.88
(3.15) |
94.97
(11.56) |
10.80
(4.97) |
17.98
(8.51) |
13.00
(7.13) |
86.95
(11.2) |
7.00 |
1.07
(1.42) |
53.00
(51.23) |
48.64
(36.67) |
32.50
(35.53) |
207.58
(84.18) |
16.00 |
36.00 |
401.00 |
HR, heart rate; RR, respiratory rate; WBC, white blood cell count; Lymph, lymphocyte percentage; CRP, C-reactive protein; SpO2, pulse oximeter oxygen saturation; BUN, blood urea nitrogen; Cr, creatinine level; ESR, erythrocyte sedimentation rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase; Plt, platelet count; PT, prothrombin time; PTT, partial thromboplastin time; N/R, not reported.
Normal ranges and units: WBC count (4.4×109/L–11×109/L); lymphocyte percentage (20%–40%); CRP ( < 1 mg/dL), SPO2 ( > 95%), BUN (7 mg/dL–20 mg/dL), Cr (0.84 mg/dL–1.21mg/dL), ESR ( < 20 mm/h), AST ( < 31U/L), ALT ( < 31U/L), Plt (165–415×109/L).
*These findings were reported as lymphocyte count.
**These finding were presented as median and IQR, to estimate the mean and standard deviation we considered median as mean and standard deviation to be IQR/1.35. because these variables seemed to have normal distribution.
Systolic and diastolic blood pressures were mostly low with mean ± SD of 110.46/74.46 ± 12.86/6.46 mmHg which was lower than the normal blood pressure 120/80 mm Hg. The mean ± SD of white blood cells (WBC) count was 10.8 ± 4.97×109/L and within the normal range of 4.5–11 ×109/L. After applying the Hozo et al7 method to the study by Pirjani et al,8 the estimated average and standard deviation of WBC count of all participant were 11.8 ± 4.68×109/L. Then, we compared WBC counts between dead and live mothers and the outcome is abstracted in Table 3.
Table 3.
Comparison of Expired Pregnant Mothers and Surviving Participants
|
Maternal Mortality
|
WBC, Mean (SD)
|
Lymphocyte,
n
(%)
|
SpO2,
n
(%)
|
Cr, Mean (SD)
|
AST, Mean (SD)
|
ALT, Mean (SD)
|
| No (75) |
12.75 (4.78) |
23.67 (8.96) |
92.25 (6.7) |
3.3323 |
72.67 (53) |
113.77 (147) |
| Yes (26) |
11.94 (3.26) |
7.63 (1.68) |
76.38 (8.69) |
1.11 (0.6) |
45.76 (20.5) |
40.62 (19.2) |
SD, standard deviation; WBC, white blood count; SpO2, oxygen saturation; Cr, creatinine; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Numbers are presented as Mean (standard deviation) or number (%).
Average WBC count in patients who expired due to COVID-19 was 11.94×109/L in comparison to 12.75×109/L among survivors. The study by Sattari et al22 was excluded from this comparison because it did not report this variable separately for live and dead patients. Also, the mean ± SD of lymphocyte percentage in all participant was 17.98% ± 8.51 and it was lower than normal (20–40%). Interestingly, lymphocyte percentage was lower in deceased patients with the mean ± SD of 7.63% ± 1.68 versus 23.67% ± 8.96 in survivors.
The mean ± SD of O2 saturation percentage was 86.95 ± 11.2% which was low. Deceased mothers also had lower SPO2 with the mean ± SD of 76.38 ± 8.69 versus 92.25% ± 6.7 in survivors. The mean ± SD of creatinine level (1.07 ± 1.42 mg/dL) was within the normal range. BUN and creatinine were only high in patients with reported kidney injuries like the patient with acute kidney injury (AKI) and acute tubular necrosis reported by Taghizadieh et al.17
The mean ± SD of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were 48.64 ± 36.67 IU/L and 32.5 ± 35.53 IU/L, respectively. Deceased patients had much lower levels of AST and ALT than survivors. Average AST among deceased participants was 45.76 ± 20.5 IU/L versus 72.67 ± 53 IU/L in survivors. The mean ± SD of ALT in deceased patients was 40.62 ± 19.2IU/L versus 113.77 ± 147 IU/L in survivors (Table 3). The prothrombin time (PT), partial thromboplastin time (PTT) and D-dimer levels were also measured in our study but unfortunately, the published data was not adequate to report.
Acute respiratory distress syndrome (ARDS) (n = 19, 12.5%), coagulopathies (n = 11, 7.2%) and cardiovascular problems (n = 8, 5%) were the most common complications that our participants experienced. Cardiovascular complications mostly consisted of cardiopulmonary collapse and right heart failure. At least 6 out of 8 patients who developed cardiovascular complications died due to COVID-19. The dead/live status of two other patients in the study by Sattari et al
22
was not reported. Common complications and prescribed medications have been summarized in Tables S2 and S3, respectively (see Supplementary file 1).
8,12-22
Neonatal Complications
Eight studies including seven case reports and one case series were selected that determined the neonatal complication of SARS-CoV-2 in newborns born to infected mothers. The characteristics of these selected studies have been summarized in Table 4. Totally, 17 neonates were included in this study. The female/male ratio was 7/10 and the mean ± SD of gestational age was 33.09 ± 3.86 weeks. Most of the neonates were preterm at the time of delivery (82.3%). The mean ± SD of birth weight was 2047.19 ± 971.57 grams.
Table 4.
Characteristics of Included Neonates to Determine the Neonatal Outcomes
|
First Author
|
Gender
|
Neonatal Mortality
|
GA at Delivery
|
Birth Weight
|
Apgar1
|
Apgar5
|
DOL at Discharge
|
Delivery
|
CS due to COVID-19
|
PCR
|
|
Female
|
Male
|
C/S
|
NVD
|
| Bordbar24 |
0 |
1 |
0 |
Term |
4300.0 |
8.00 |
9.00 |
4.00 |
1 |
0 |
0 |
1 |
| Dorgalaleheh9 |
0 |
1 |
0 |
Term |
N/R |
N/R |
N/R |
16.00 |
1 |
0 |
0 |
1 |
| Farsi,11 case 1 |
1 |
0 |
1 |
30.00 |
1320.0 |
4.00 |
7.00 |
Died |
1 |
0 |
0 |
0 |
| Farsi, case 2 |
0 |
1 |
0 |
30.00 |
1600.0 |
5.00 |
7.00 |
37.00 |
1 |
0 |
0 |
1 |
| Farsi, case 3 |
1 |
0 |
1 |
30.00 |
1200.0 |
6.00 |
7.00 |
Died |
1 |
0 |
0 |
0 |
| Parsa25 |
1 |
0 |
0 |
37.00 |
3500.0 |
9.00 |
10.00 |
28.00 |
1 |
0 |
0 |
1 |
| Rashidiann26 |
0 |
1 |
1 |
39.00 |
3545.0 |
9.00 |
10.00 |
1.00 |
1 |
0 |
0 |
0 |
| Sagheb,23 case 1 |
0 |
1 |
0 |
31.00 |
1550.0 |
7.00 |
8.00 |
60.00 |
1 |
0 |
0 |
1 |
| Sagheb, case 2 |
0 |
1 |
0 |
33.50 |
1930.0 |
7.00 |
8.00 |
N/R |
1 |
0 |
1 |
1 |
| Schwartz,10 case 1 |
0 |
1 |
0 |
34.00 |
1800.0 |
4.00 |
5.00 |
7.00 |
1 |
0 |
0 |
1 |
| Schwartz, case 2 |
0 |
1 |
0 |
31.00 |
1660.0 |
7.00 |
8.00 |
21.00 |
0 |
1 |
0 |
1 |
| Schwartz, case 3 |
1 |
0 |
1 |
28.00 |
900.0 |
N/R |
N/R |
Died |
1 |
0 |
0 |
1 |
| Schwartz, case 4 |
1 |
0 |
0 |
34.00 |
2000.0 |
9.00 |
10.00 |
7.00 |
1 |
0 |
1 |
1 |
| Schwartz, case 5 |
1 |
0 |
0 |
32.00 |
2300.0 |
5.00 |
5.00 |
12.00 |
1 |
0 |
1 |
1 |
| Schwartz, case 6 |
0 |
1 |
0 |
28.00 |
900.0 |
N/R |
N/R |
N/R |
0 |
0 |
0 |
1 |
| Schwartz, case 7 |
0 |
1 |
0 |
33.00 |
1900.0 |
7.00 |
7.00 |
14.00 |
1 |
0 |
1 |
1 |
| Zamaniyan18 |
1 |
0 |
0 |
32.00 |
2350.0 |
8.00 |
9.00 |
.00 |
1 |
0 |
1 |
1 |
|
Total
|
7
(41) |
10
(59) |
4 (23.5) |
33.09
(3.86) |
2047.19
(971.57) |
6.79
(1.76) |
7.86
(1.65) |
20.60
(17.19) |
15 (88.2) |
1 (0.05) |
5 (29.4) |
14 (82.3) |
GA, gestational age; DOL, day of life; CS, caesarean section; NVD, normal vaginal delivery; PCR, polymerase chain reaction.
Total is presented as number (percentage) or Mean (standard deviation).
Five neonates had an Apgar score lower than 7 one minute after delivery while only one neonate had an Apgar score lower than 7 five minutes after delivery. The mean ± SD of Apgar score one and five minutes after birth were 6.79 ± 1.76 and 7.86 ± 1.65, respectively. SARS-CoV-2 specific RT-PCR test was positive in 14 neonates. Fifteen neonates were delivered by cesarean section and in at least five patients, COVID-19 was reported as the main culprit. The neonatal mortality rate was 4 out of 17 (23.5%).
Table S4 of Supplementary file 1 summarizes the clinical findings and symptoms of the newborns.
9,10,11,18,23-26
Eleven (64.7%) neonates were transferred to NICU after delivery and 14 (82.3%) neonates developed respiratory distress syndrome (RDS). No case of sepsis was reported and we did not observe any other significant adverse complications among them except two cases of pulmonary hemorrhage reported by Sagheb et al and Rashidian et al.
26
Ten neonates had COVID-19-related findings in their CT-scan or chest X-ray (CXR). Laboratory finding of the newborns is listed in Table S5.
9,10,23,24
Four patients had low lymphocyte counts and other significant changes were not reported in other tests.
Vertical Transmission
Periodical neonatal RT-PCR tests plus vaginal secretions, umbilical cord blood and amniotic fluid and neonates’ urine, feces and gastric juice tests for SARS-CoV-2 antigens were used to determine the possibility of vertical transmission of SARS-CoV-2. Totally, 14 newborns had positive RT-PCR test. Three newborns were positive on day 1, whereas six neonates had a negative result on day 1 (Table S6).9-11,18,23-26 On the average, the neonate’s PCR test became positive on day 4 for the first time. Amniotic fluid test was positive in three mothers and SARS-CoV-2 antigens were also detected in vaginal secretion of an infected mother. Eleven neonates were transferred to NICU and skin to skin contract was limited. The cases reported by Dorgalale et al.9 discharged after birth had contact with their mother for half a day.
Discussion
In this review, the most common symptoms among pregnant women included fever, cough, and myalgia. However, kidney injuries, cardiovascular complication and coagulation problems were the most important complications that caught our attention. It was found that pregnant women who expired or developed IUFD had an average lower SpO2, indicating that women with more severe lung involvement were at higher risk of maternal and neonate mortality than those with mild lung involvement. This finding is consistent with a study by Turan et al.27 Therefore, it seems that pregnant women with severe symptoms and low oxygen saturation need special care, while women with mild symptoms usually do not face serious problems.
Pregnant women are susceptible to AKI.28 Additionally, coronavirus could cause kidney injuries.29 AKI was present in three patients two of whom expired. In the case reported by Taghizadeh et al,17 a pregnant mother developed acute tubular necrosis, and the most possible reason to explain her condition was COVID-19. Fortunately, this patient was treated by hemodialysis. Kidney injuries were associated with an increase in creatinine levels while creatinine level was normal in other patients who did not experience renal involvement. It seems that in severe illness, AKI was more severe than in mild patients and BUN and creatinine levels were also higher in the first group. Also, another study reported three kidney injuries in 99 people.30 On the contrary, the study by Wang et al on 116 patients did not find any relation between COVID-19 and AKI.31 Finally, it can be concluded that even if pregnancy with COVID-19 and kidney injuries are related, pregnancy does not seem to have a major impact on the incidence of kidney injuries related to COVID-19. However, more studies are required to make a reliable statement. Nevertheless, hemodynamic monitoring of pregnant women with COVID-19 is necessary.
COVID-19 is associated with myocarditis, ACS, heart failure and arrhythmia. Systemic inflammation following COVID-19 may causes these cardiovascular consequences in patients.32 In our study, eight pregnant women had cardiovascular problems, six of whom expired. In a case-series reported by Hantoushzadeh et al, four patient developed cardiopulmonary collapse, none of whom had a history of underlying disease associated with cardiovascular problems.20 In a study by Karami et al, a pregnant mother with COVID-19 was diagnosed with right heart hypertrophy,13 which may be due to increased pulmonary artery pressure. In a study on 799 patients with COVID-19 in Wuhan, heart failure was found in 24% of all patients and 49% of those who died.33 Another study on 138 patients reported 17% arrhythmias in COVID-19 patients.34 Considering the death rate of 75% for patients who had cardiovascular problems in our study, it seems that cardiovascular problems are a risk factor for the poor prognosis of COVID-19 in pregnant women.
One of the cases that caught our attention was in the study by Mohammadi et al who reported an ovarian vein thrombosis in a pregnant woman with COVID-19.15 It is well known that pregnancy is a risk factor for increased coagulation. In addition, several studies have shown the link between COVID-19 and coagulation problems. For example, according to Nishiga et al, coagulation complications were more common in patients with COVID-19 but mostly did not meet the criteria of disseminated intravascular coagulation (DIC). The systemic inflammation and hypercoagulable state may be the cause of these coagulation complications.32 In our study, there were eleven patients with coagulopathies (three with DIC, three with thrombosis or emboli and five others whose exact condition was not reported).
Platelet counts were normal in all patients but the average platelet count was a little bit higher in patients who experienced coagulation problems, although within the normal range. In a review article by Wool et al, they found that COVID-19 patients had a slightly higher platelet count in comparison to the control group.35 Unfortunately, we did not have enough data for D-dimer levels, PT and PTT, so we cannot judge conclusively. Nevertheless, we recommend risk assessment for coagulopathies like DVT and imaging modalities like MRA and CTA for pregnant women with COVID-19.
Fourteen preterm deliveries were reported out of the 17 included neonates, which is significantly high. In a recent systematic review by Di Toro et al, the prevalence of preterm delivery was reported to be 23% among 684 newborn.36 This difference could be due to the small number of participants in our study or selection bias. But we should not miss the fact that most of the included mothers in our study had symptomatic SARS-CoV-2 infection and two maternal deaths were reported. Still birth and preterm delivery rates have increased during the SARS-CoV-2 pandemic.37 Also, maternal COVID-19 could somehow cause preterm delivery. Mean birthweight was also low and this finding was due to preterm delivery plus a triplet reported by Zamaniyan et al with low birthweight.
RDS was the most common finding among included neonates which is consistent with previous studies.38 Lymphocytopenia was found in three newborns. In a cohort study in the US, none of the neonates had considerable complications and all RT-PCR tests resulted negative.39 It should be considered that 14 newborns in our study had positive RT-PCR tests indicating that lymphocytopenia and RDS may be secondary to SARS-CoV-2 infection among these neonates. Interestingly, anuria was reported in one,11 and pulmonary bleeding was reported in two neonates23,26 which were not mentioned in other studies. Overall, it seems that maternal COVID-19 does not have a direct neonatal impact other than preterm delivery or fetal distress (not found in our study) and the prevention strategies to reduce the risk of mother to infant transmission should be considered.40
Three neonates had positive RT-PCR test on the first day of life and three others were positive on day two. On the average, the neonate’s PCR became positive on day 4 for the first time. Raschetti et al41 suggested a vertical transmission with probability of 30%. The results of the current study show that even after excluding participants whose skin to skin contact protections and breastfeeding protocols were not reported, the risk of vertical transmission is still more considerable than our current expectations. But more studies are required to confirm this finding. Nevertheless, what is for sure is the need for extra care for SARS-CoV-2 infected pregnant women and adoption of alternative strategies for breastfeeding and adhering to protection protocols.
The included articles only belong to one country and our findings could be used to compare outcomes from different parts of the world. Case reports are mostly about special patients with special complications, and the Hantoushzadeh et al20 case series was not a consecutive case series and only expired pregnant women were included in that study. The cohort study by Amini Moghadam et al 21 also had the same problems and their participants were not randomly selected. Up to the present time, two cohort studies by Sattari et al22 and Pirjani et al8 have selected random cases. One of the major limitations was the fact that many variables were not mentioned in all of the articles.
In conclusion, early diagnosis of COVID-19 disease is crucial in pregnant women, because there is a possibility of worsening complications in the mother and fetus, and also to use the prevention strategies to reduce the risk of mother to infant transmission.
Supplementary Materials
Supplementary file 1 contains Tables S1-S6.
(pdf)
Acknowledgements
We appreciate the efforts of all the researchers whose articles were included in this study.
Authors’ Contribution
ZH: Project development. MG: Manuscript editing. BHR: Project development. OK and HF: Data collection and management. MZ: Data management and manuscript writing.
Conflict of Interest Disclosures
None.
Ethical Statement
This manuscript was performed in accordance with Helsinki declaration. All patients’ data were kept confidential. This study was approved by Tehran University of Medical Science’s ethical committee.
References
- World Health Organization. Coronavirus Disease ( COVID-19): Weekly Epidemiological Update. 11 October 2020.
- Hopkins J. Johns Hopkins Coronavirus Resource Center. Available from: https://coronavirus.jhu.edu/data/mortality. Accessed October 18, 2020.
- Liu H, Wang LL, Zhao SJ, Kwak-Kim J, Mor G, Liao AH. Why are pregnant women susceptible to COVID-19? an immunological viewpoint. J Reprod Immunol 2020; 139:103122. doi: 10.1016/j.jri.2020.103122 [Crossref] [ Google Scholar]
- Tan EK, Tan EL. Alterations in physiology and anatomy during pregnancy. Best Pract Res Clin Obstet Gynaecol 2013; 27(6):791-802. doi: 10.1016/j.bpobgyn.2013.08.001 [Crossref] [ Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [Crossref] [ Google Scholar]
- Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute; 2017.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. doi: 10.1186/1471-2288-5-13 [Crossref] [ Google Scholar]
- Pirjani R, Hosseini R, Soori T, Rabiei M, Hosseini L, Abiri A. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J Travel Med 2020; 27(7):taaa158. doi: 10.1093/jtm/taaa158 [Crossref] [ Google Scholar]
- Dorgalaleh A, Baghaipour MR, Tabibian S, Ghazizadeh F, Dabbagh A, Bahoush G. Gastrointestinal bleeding in a newborn infant with congenital factor X deficiency and COVID-19- A common clinical feature between a rare disorder and a new, common infection. Int J Lab Hematol 2020; 42(6):e277-e9. doi: 10.1111/ijlh.13318 [Crossref] [ Google Scholar]
- Schwartz DA, Mohagheghi P, Beigi B, Zafaranloo N, Moshfegh F, Yazdani A. Spectrum of neonatal COVID-19 in Iran: 19 infants with SARS-CoV-2 perinatal infections with varying test results, clinical findings and outcomes. J Matern Fetal Neonatal Med 2020:1-10. doi: 10.1080/14767058.2020.1797672 [Crossref]
- Farsi Z, Taheri Derakhsh N, Bassirnia M, Ahmadi L, Shiva M, Yousefzadegan S. Coronavirus disease-2019 infection in neonates of an infected pregnant mother with triplets. Iran J Neonatol 2020; 11(3):120-2. doi: 10.22038/ijn.2020.49218.1856 [Crossref] [ Google Scholar]
- Jafari R, Jonaidi-Jafari N, Dehghanpoor F, Saburi A. Convalescent plasma therapy in a pregnant COVID-19 patient with a dramatic clinical and imaging response: a case report. World J Radiol 2020; 12(7):137-41. doi: 10.4329/wjr.v12.i7.137 [Crossref] [ Google Scholar]
- Karami P, Naghavi M, Feyzi A, Aghamohammadi M, Novin MS, Mobaien A. WITHDRAWN: Mortality of a pregnant patient diagnosed with COVID-19: a case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis 2020:101665. doi: 10.1016/j.tmaid.2020.101665 [Crossref]
- Khodamoradi Z, Sadeghi Boogar S, Kamali Haghighi Shirazi F, Kouhi P. COVID-19 and acute pulmonary embolism in postpartum patient. Emerg Infect Dis 2020; 26(8):1937-9. doi: 10.3201/eid2608.201383 [Crossref] [ Google Scholar]
- Mohammadi S, Abouzaripour M, Hesam Shariati N, Hesam Shariati MB. Ovarian vein thrombosis after coronavirus disease (COVID-19) infection in a pregnant woman: case report. J Thromb Thrombolysis 2020; 50(3):604-7. doi: 10.1007/s11239-020-02177-6 [Crossref] [ Google Scholar]
- Soleimani Z, Soleimani A. ADRS due to COVID-19 in midterm pregnancy: successful management with plasma transfusion and corticosteroids. J Matern Fetal Neonatal Med 2020:1-4. doi: 10.1080/14767058.2020.1797669 [Crossref]
- Taghizadieh A, Mikaeili H, Ahmadi M, Valizadeh H. Acute kidney injury in pregnant women following SARS-CoV-2 infection: a case report from Iran. Respir Med Case Rep 2020; 30:101090. doi: 10.1016/j.rmcr.2020.101090 [Crossref] [ Google Scholar]
- Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat Diagn 2020; 40(13):1759-61. doi: 10.1002/pd.5713 [Crossref] [ Google Scholar]
- Gheysarzadeh A, Sadeghifard N, Safari M, Rashidian T, Mohammadyari E, Tavan H. Case series of four pregnant women with COVID-19 in Ilam, Iran. New Microbes New Infect 2020; 38:100783. doi: 10.1016/j.nmni.2020.100783 [Crossref] [ Google Scholar]
- Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, Seferovic MD, Aski SK, Arian SE. Maternal death due to COVID-19. Am J Obstet Gynecol 2020; 223(1):109.e1-109. doi: 10.1016/j.ajog.2020.04.030 [Crossref] [ Google Scholar]
- Amini Moghadam S, Dini P, Nassiri S, Motavaselian M, Hajibaba M, Sohrabi M. Clinical features of pregnant women in Iran who died due to COVID-19. Int J Gynaecol Obstet 2021; 152(2):215-9. doi: 10.1002/ijgo.13461 [Crossref] [ Google Scholar]
- Sattari M, Bashirian S, Masoumi SZ, Shayan A, Jenabi E, Ghelichkhani S. Evaluating clinical course and risk factors of infection and demographic characteristics of pregnant women with COVID-19 in Hamadan province, west of Iran. J Res Health Sci 2020; 20(3):e00488. doi: 10.34172/jrhs.2020.22 [Crossref] [ Google Scholar]
- Sagheb S, Lamsehchi A, Jafary M, Atef-Yekta R, Sadeghi K. Two seriously ill neonates born to mothers with COVID-19 pneumonia- a case report. Ital J Pediatr 2020; 46(1):137. doi: 10.1186/s13052-020-00897-2 [Crossref] [ Google Scholar]
- Bordbar A, Kashaki M, Rezaei F, Jafari R. Vertical transmission of COVID-19 in a 1-day-old neonate. Travel Med Infect Dis 2020; 38:101879. doi: 10.1016/j.tmaid.2020.101879 [Crossref] [ Google Scholar]
- Parsa Y, Shokri N, Jahedbozorgan T, Naeiji Z, Zadehmodares S, Moridi A. Possible vertical transmission of COVID-19 to the newborn; a case report. Arch Acad Emerg Med 2021; 9(1):e5. doi: 10.22037/aaem.v9i1.923 [Crossref] [ Google Scholar]
- Rashidian T, Sharifi N, Fathnezhad-Kazemi A, Mirzamrajani F, Nourollahi S, Ghaysouri A. Death of a neonate with suspected coronavirus disease 2019 born to a mother with coronavirus disease 2019 in Iran: a case report. J Med Case Rep 2020; 14(1):186. doi: 10.1186/s13256-020-02519-1 [Crossref] [ Google Scholar]
- Turan O, Hakim A, Dashraath P, Jeslyn WJL, Wright A, Abdul-Kadir R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: a systematic review. Int J Gynaecol Obstet 2020; 151(1):7-16. doi: 10.1002/ijgo.13329 [Crossref] [ Google Scholar]
- Jim B, Garovic VD. Acute kidney injury in pregnancy. Semin Nephrol 2017; 37(4):378-85. doi: 10.1016/j.semnephrol.2017.05.010 [Crossref] [ Google Scholar]
- Li Z, Wu M, Yao J, Guo J, Liao X, Song S, et al. Caution on kidney dysfunctions of COVID-19 patients. medRxiv. 2020. 10.1101/2020.02.08.20021212
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395(10223):507-13. doi: 10.1016/s0140-6736(20)30211-7 [Crossref] [ Google Scholar]
- Wang L, Li X, Chen H, Yan S, Li D, Li Y. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol 2020; 51(5):343-8. doi: 10.1159/000507471 [Crossref] [ Google Scholar]
- Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020; 17(9):543-58. doi: 10.1038/s41569-020-0413-9 [Crossref] [ Google Scholar]
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368:m1091. doi: 10.1136/bmj.m1091 [Crossref] [ Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323(11):1061-9. doi: 10.1001/jama.2020.1585 [Crossref] [ Google Scholar]
- Wool GD, Miller JL. The impact of COVID-19 disease on platelets and coagulation. Pathobiology 2021; 88(1):15-27. doi: 10.1159/000512007 [Crossref] [ Google Scholar]
- Di Toro F, Gjoka M, Di Lorenzo G, De Santo D, De Seta F, Maso G. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect 2021; 27(1):36-46. doi: 10.1016/j.cmi.2020.10.007 [Crossref] [ Google Scholar]
- Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA 2020; 324(7):705-6. doi: 10.1001/jama.2020.12746 [Crossref] [ Google Scholar]
- Vardhelli V, Pandita A, Pillai A, Badatya SK. Perinatal COVID-19: review of current evidence and practical approach towards prevention and management. Eur J Pediatr 2021; 180(4):1009-31. doi: 10.1007/s00431-020-03866-3 [Crossref] [ Google Scholar]
- Salvatore CM, Han JY, Acker KP, Tiwari P, Jin J, Brandler M. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health 2020; 4(10):721-7. doi: 10.1016/s2352-4642(20)30235-2 [Crossref] [ Google Scholar]
- De Rose DU, Piersigilli F, Ronchetti MP, Santisi A, Bersani I, Dotta A. Novel coronavirus disease (COVID-19) in newborns and infants: what we know so far. Ital J Pediatr 2020; 46(1):56. doi: 10.1186/s13052-020-0820-x [Crossref] [ Google Scholar]
- Raschetti R, Vivanti AJ, Vauloup-Fellous C, Loi B, Benachi A, De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun 2020; 11(1):5164. doi: 10.1038/s41467-020-18982-9 [Crossref] [ Google Scholar]