Arch Iran Med. 24(12):919-920.
doi: 10.34172/aim.2021.138
Photoclinic
Menkes Disease
Mahmoud Reza Ashrafi 1  , Dorsa Ghasemi 2, Moeinadin Safavi 2, *
, Dorsa Ghasemi 2, Moeinadin Safavi 2, * 
Author information:
1Department of Pediatric Neurology, Growth and Development Research Center, Children’s Medical Center, Pediatrics Center of Excellence, Tehran University of Medical Sciences, Tehran, Iran
2Pathology Department, Children’s Medical Center, Tehran University of Medical Sciences, Tehran, Iran
*Corresponding Author: Moeinadin Safavi, MD; Pathology Department, Children’s Medical Center, Tehran University of Medical Sciences, Tehran, Iran. Tel: +98-21-61472405; Fax: +98-21-66948780; Email:
moein.safavi@gmail.com
Copyright and License Information
© 2021 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Ashrafi MR, Ghasemi D, Safavi M. Photoclinic. Arch Iran Med. 2021;24(12):919-920. doi: 10.34172/ aim.2021.138
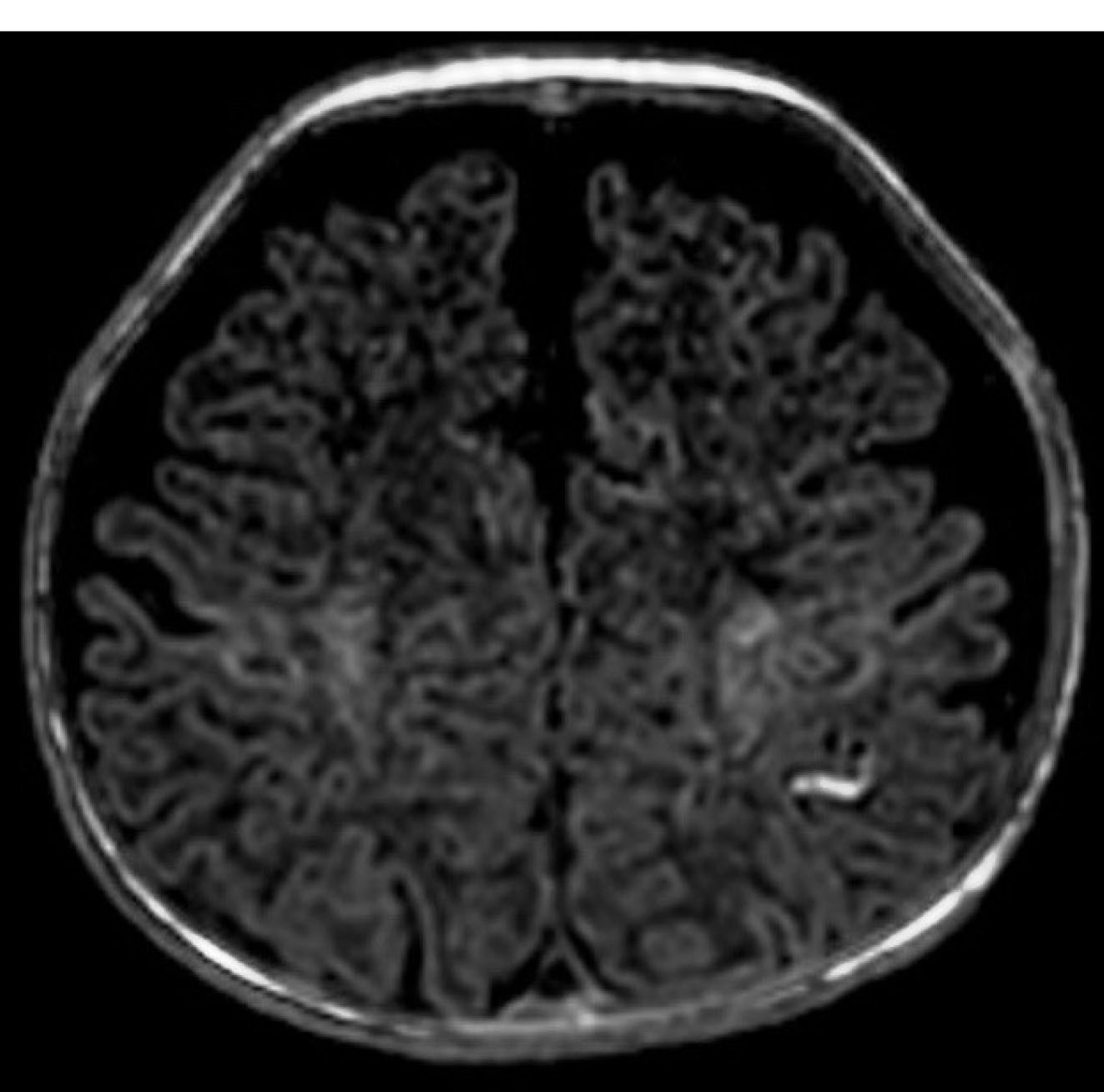
Figure 1.
Brain MRI (T1W) revealed mildly delayed myelination, benign subarachnoid space enlargement, and a vascular anomaly in the left parietal lobe.
.
Brain MRI (T1W) revealed mildly delayed myelination, benign subarachnoid space enlargement, and a vascular anomaly in the left parietal lobe.
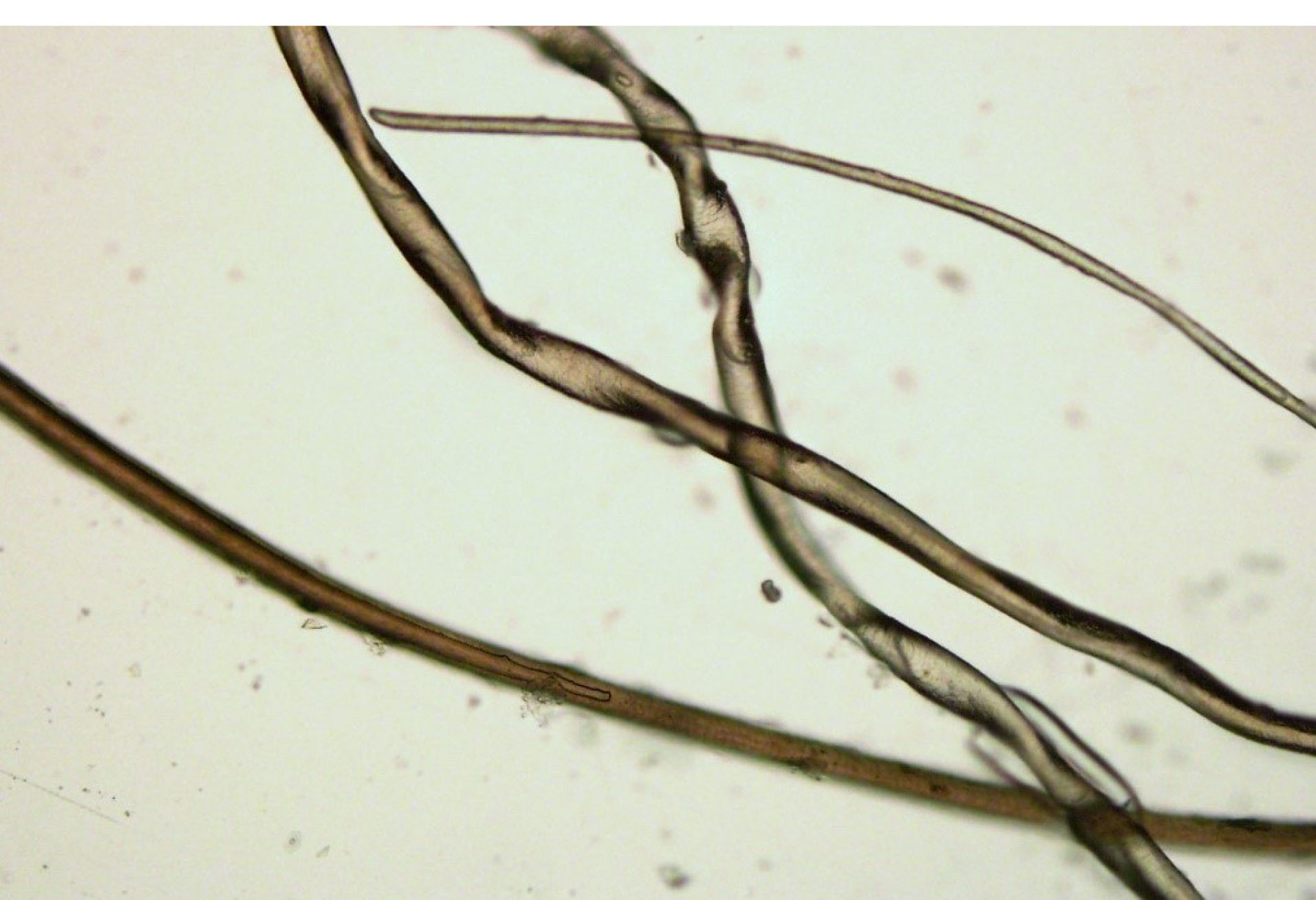
Figure 2.
Microscopic examination of the hair showed pili torti, hair shaft thinning, and decreased pigmentation.
.
Microscopic examination of the hair showed pili torti, hair shaft thinning, and decreased pigmentation.
The patient was a 2-month-old male infant with seizure, hypotonia, poor feeding, and vomiting. He had fair complexion and hypopigmented sparse woolly hair. Brain magnetic resonance imaging (MRI) revealed a mild delay in myelination and benign subarachnoid space enlargement with 6.5 mm craniocortical and 10.5 mm interhemispheric diameter. It also exhibited cortical hyperintensity in T1-weighted sequences (T1W) in the left partial lobe that could be due to vascular anomaly (Figure 1). Laboratory tests were unremarkable except for low serum ceruloplasmin (100 mg/L, normal range 200-600 mg/L). Hair microscopy revealed pili torti, hair shaft thinning, and decreased pigmentation (Figure 2).
Photoclinic Diagnosis
Menkes disease (MD) is characterized by copper deficiency in contrast with Wilson’s disease. MD is a rare X-linked genetic disorder caused by ATP7A mutations with a frequency of 0.8–2/100 000 live male births. Its pathogenesis is explained by the defective activity of several essential copper-containing metalloenzymes like cytochrome c oxidase, lysyl oxidase, superoxide dismutase, dopamine β hydroxylase, ascorbic acid oxidase, and tyrosinase.1,2
Classical clinical manifestations of MD that begin from the neonatal period include hypothermia, hypoglycemia, poor feeding, impaired weight gain, and less often hemorrhagic diathesis like cephalohematoma. Later, at the age of 2–3 months, MD presents with seizure, failure to thrive, and developmental delay. The most striking finding is colorless and friable hair. Microscopic examination of the hair commonly exhibits pili torti that is the hair shaft flattening and twisting 180 degrees on its axis.1,3,4
Neuroimaging studies frequently show impaired myelination, cerebral atrophy, regions of low density within the cortex, tortuous and enlarged intracranial vessels, and subdural hematoma. MD diagnosis is suggested by typical presentations like seizure, developmental delay, and unusual hair. Finally, it is diagnosed by reduced levels of serum ceruloplasmin, and copper. However, serum copper and ceruloplasmin are within normal limits during the first 6 weeks of life and cannot be utilized diagnostically very early. Abnormal catecholamine metabolites (due to dopamine β hydroxylase deficiency) can be utilized as a sensitive and specific diagnostic test and may contribute to earlier diagnosis of MD.5
Copper replacement therapy with daily subcutaneous injection of copper histidine is the treatment of MD and might have a relatively satisfactory outcome if initiated in the early days of life. Nevertheless, the overall prognosis is poor and death happens in the first 3 years of life.1,6
Authors’ Contribution
MA: Involved in patient clinical management and drafting the manuscript. MS and DG: Involved in pathology interpretation, image preparation and drafting the manuscript.
Conflict of Interest Disclosures
The authors have no conflicts of interest.
Ethical Statement
Informed consent was obtained from the patient’s parents.
References
- Rosenberg RN, Pascual JM. Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease: Volume 1. Academic Press; 2020. p. 613-21.
- Harrison MD, Dameron CT. Molecular mechanisms of copper metabolism and the role of the Menkes disease protein. J Biochem Mol Toxicol 1999; 13(2):93-106. doi: 10.1002/(sici)1099-0461(1999)13:2<93::aid-jbt5>3.0.co;2-3 [Crossref] [ Google Scholar]
- Ahmed A, Almohanna H, Griggs J, Tosti A. Genetic hair disorders: a review. Dermatol Ther (Heidelb) 2019; 9(3):421-48. doi: 10.1007/s13555-019-0313-2 [Crossref] [ Google Scholar]
- Mirmirani P, Huang KP, Price VH. A practical, algorithmic approach to diagnosing hair shaft disorders. Int J Dermatol 2011; 50(1):1-12. doi: 10.1111/j.1365-4632.2010.04768.x [Crossref] [ Google Scholar]
- Goldstein DS, Holmes CS, Kaler SG. Relative efficiencies of plasma catechol levels and ratios for neonatal diagnosis of Menkes disease. Neurochem Res 2009; 34(8):1464-8. doi: 10.1007/s11064-009-9933-8 [Crossref] [ Google Scholar]
- Tang J, Donsante A, Desai V, Patronas N, Kaler SG. Clinical outcomes in Menkes disease patients with a copper-responsive ATP7A mutation, G727R. Mol Genet Metab 2008; 95(3):174-81. doi: 10.1016/j.ymgme.2008.06.015 [Crossref] [ Google Scholar]