Arch Iran Med. 25(1):37-49.
doi: 10.34172/aim.2022.07
Guideline
Iranian Consensus Recommendations for Treatment of Myasthenia Gravis
Shahriar Nafissi 1, *  , Ali Asghar Okhovat 1, 2
, Ali Asghar Okhovat 1, 2  , Farnaz Sinaei 1, Behnaz Ansari 3, Hormoz Ayramloo 4, Keyvan Basiri 3, Reza Boostani 5, Bahram Haghi Ashtiani 6, Payam Sarraf 7, Farzad Fatehi 1
, Farnaz Sinaei 1, Behnaz Ansari 3, Hormoz Ayramloo 4, Keyvan Basiri 3, Reza Boostani 5, Bahram Haghi Ashtiani 6, Payam Sarraf 7, Farzad Fatehi 1 
Author information:
1Neurology Department, Shariati Hospital, Iranian Neuromuscular Research Center (INMRC), Tehran University of Medical Sciences, Tehran, Iran
2Neurology Department, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
3Department of Neurology, Isfahan University of Medical Sciences, Isfahan, Iran
4Department of Neurology, Tabriz University of Medical Sciences, Tabriz, Iran
5Neurology Department, Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran
6Department of Neurology, Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran
7Neurology Department, Imam-Khomeini Complex Hospital, Tehran University of Medical Sciences, Tehran, Iran
*Corresponding Author: Shahriar Nafissi, MD; Neuromuscular Department, Shariati Hospital, Tehran University of Medical Sciences, Iran. Tel:+98-21-84902224; Fax:+98-21 88633039; Email:
nafisi@tums.ac.ir,
nafissishahriar@gmail.com
Abstract
Myasthenia gravis (MG) is an immune-mediated potentially treatable disease in which rapid diagnosis and proper treatment can control symptoms. Treatment should be individualized in each patient according to distribution (ocular or generalized) and severity of the weakness, antibody status, thymus pathology, patient comorbidities, and preferences. A group of Iranian neuromuscular specialists have written these recommendations to treat MG based on national conditions. Four of the authors performed an extensive literature review, including PubMed, EMBASE, and Google Scholar, from 1932 to 2020 before the central meeting to define headings and subheadings. The experts held a 2-day session where the primary drafts were discussed point by point. Primary algorithms for the management of MG patients were prepared in the panel discussion. After the panel, the discussions continued in virtual group discussions, and the prepared guideline was finalized after agreement and concordance between the panel members. Finally, a total of 71 expert recommendations were included. We attempted to develop a guideline based on Iran’s local requirements. We hope that these guidelines help healthcare professionals in proper treatment and follow-up of patients with MG.
Keywords: Consensus, Myasthenia Gravis, Iran, Therapy
Copyright and License Information
© 2022 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Nafissi S, Okhovat AA, Sinaei F, Ansari B, Ayramloo H, Basiri K, et al. Iranian consensus recommendations for treatment of myasthenia gravis. Arch Iran Med. 2022;25(1):37-49. doi: 10.34172/aim.2022.07
Introduction
Myasthenia gravis (MG) is an auto-immune neuromuscular junction disorder manifested by weakness of the extraocular, limb, bulbar, and respiratory muscles. Its prevalence is approximately 1 in 5000. Although MG can affect patients of all ages, the prevalence is higher in young women and older men. Over the past 50 years, an increasing number of patients are being diagnosed due to better diagnostic tools and more patients are surviving due to improved treatment options and increased life expectancy.1
Treatment should be individualized in each patient according to distribution (ocular or generalized) and severity of the weakness, antibodies status, thymus pathology, patient comorbidities, and patient preferences.2,3 For MG patients refractory to conventional immunosuppression therapy, biologic agents such as monoclonal antibodies are highly promising.4–6 A group of Iranian neuromuscular specialists have written these recommendations for the treatment of MG based on national conditions.
Diagnosis
Based on age, the presence and type of antibodies, the pathology of the thymus, and the region of muscles involved in MG, the patients are divided into the following categories:
-
Ocular MG
-
Early-onset generalized non-thymomatous ACR-positive MG
-
Late-onset generalized non-thymomatous ACR-positive MG
-
Thymomatous MG
-
Muscle-specific tyrosine kinase (MuSK)-positive MG
-
Generalized seronegative MG
MG is assumed in patients complaining of diurnal variation of muscle weakness. When there is a clinical diagnosis of MG, the diagnosis should be confirmed by paraclinical assays. These include electrodiagnostic studies (EDX), pharmacologic testing, and serum antibodies assay. EDX includes slow (2 to 5-Hz) repetitive nerve stimulation (RNS) and single-fiber electromyography (SFEMG). EDX is beneficial in double-seronegative MG patients.7 The most sensitive test for diagnosis of MG is SFEMG, and, when studied in weak muscles, positive results are recorded in nearly all patients. However, an abnormal SFEMG (increased jitter or blocking) is not specific for MG and, apart from other diseases of the neuromuscular junction, can be found in motor neuron disease or myopathies.8
A positive response to ChEIs (Tensilon test), as unambiguous quantifiable muscle weakness improvement, strongly supports MG’s diagnosis although it is not specific. The positive responses to the edrophonium test are around 90% in MG.9 In MuSK MG, ChEI injection is often unsuccessful, provokes cramp and fasciculations, and may even induce clinical worsening.10 A positive Tensilon test can be seen in congenital myasthenic syndromes, Lambert-Eaton myasthenic syndrome, Amyotrophic lateral sclerosis (ALS), and Guillain-Barré syndrome.11
Serological testing is one of the diagnostic tools in a patient suspected of having MG. AChR-binding antibodies to MG are highly specific (97%–99%) 12 and are positive in about 80% of generalized MG patients, and 50% of patients with ocular MG.1 In very rare conditions, false-positive results have been seen including in some patients with Guillain-Barré syndrome, asymptomatic thymoma, and patients with ALS.1,13 If AChR antibodies are negative, MuSK antibodies are tested that are present in around one-third of seronegative generalized MG patients.14 In approximately 15%-20% of patients, both antibodies are negative (double seronegative). Antibodies to low-density lipoprotein receptor-related protein 4 (LRP4) and agrin have been described in some double seronegative patients.15,16
Methods
A group of neuromuscular experts with particular interest and experience in the management of MG participated in developing this guideline. Search terms were created. Four of the authors performed an extensive literature review, including PubMed, EMBASE, and Google Scholar, from 1932 to 2020 before the central meeting to define headings and subheadings. Each panel member prepared a draft on an assigned heading before the consensus session.
The experts held a 2-day session where the primary drafts were discussed point by point. Primary algorithms for the management of MG patients were prepared in the panel discussion. In case of discordance, the matter was subjected to vote and settled once more than two-thirds of the participants voted positively. After the Panel, the discussions continued in virtual group discussions, and the prepared guideline was finalized after agreement and concordance among the panel members.
Treatment
Symptomatic Treatment
The efficacy of ChEIs in treating MG has not yet been evaluated in a randomized controlled trial (RCT). However, there are observational studies, including case series and daily clinical experiences, regarding the beneficial effect of this drug in MG.17 There is only one RCT to compare the effects of intranasal neostigmine and placebo in MG with significant clinical and electromyographic improvement for intranasal neostigmine.18 Pyridostigmine is started at a 30-60 mg dose three times daily. The dosage is then gradually increased to keep the symptoms minimum, with a maximum dose of 120 mg every three hours (960 mg/d).2 For pediatric use, the initial prescription is 0.5 to 1 mg/kg, and the highest daily dose is 7 mg/kg separated into five to six doses.19,20 Improvement does not occur on pyridostigmine in some MuSK-MG patients, and standard doses may cause marked nicotinic side effects, such as diffuse fasciculations and cramps.21
In patients with mild symptoms, ChEIs may suffice as the sole treatment.2 Typically, generalized MG patients need pyridostigmine with prednisone for adequate clinical improvement.2 Corticosteroids or immunosuppressive therapy should be initiated in all patients with MG who have not received adequate pyridostigmine improvement after optimal dosing.3
Recommendations
-
ChEIs should be tried as the initial treatment for all subtypes of MG.
-
Particular caution should be made in patients with myasthenic crisis and MuSK MG.
-
All ChEIs are stopped while the patient is intubated. However, some experts believe ChEIs might be continued if excessive bronchial secretions are not a significant concern.
-
We prefer to initiate immunomodulation therapy if 240 mg of daily pyridostigmine does not achieve adequate symptom control.
-
We suggest starting pyridostigmine 30-60 mg three times daily and then level up the dose gradually to keep the symptoms to a minimum, with a maximum dose of 120 mg every three hours (960 mg).
-
Pyridostigmine dose should be adjusted as required based on symptoms. The ultimate goal of immunotherapy is to improve symptoms and minimize the required dose of symptomatic therapy.
Intravenous Immunoglobulin (IVIg) and Plasma Exchange (PLEX)
Several studies have demonstrated the efficacy of IVIg22–29 and PLEX30-43 in MG. They have been used both in the MG crisis or exacerbation,27,28 as maintenance therapies,24,25,44 in patients who do not tolerate corticosteroids or other steroid-sparing drugs or when there is a contraindication.45 The choice of IVIg or PLEX depends on general information of the disorders, the therapeutic effects, and the unwanted side effects of each of these approaches.46 A randomized trial in 1997 comparing IVIg and PLEX showed that the myasthenic severity score variation was comparable in both groups; however, IVIg tolerance was significantly better than PLEX.47 In another retrospective study on patients with MG crisis (in 54 crises), PLEX was considerably better in ventilatory improvement at two weeks and one-month functional outcome, although with a higher complication rate.48
In another study, the efficacy of PLEX vs. IVIg was compared in moderate to severe MG (n = 84),49 and both were equally effective in reducing QMGS. In the meta-analysis (10 articles eligible for the analysis for MG patients), PLEX or IVIg were similar in terms of efficacy or safety and hospital stay length and ventilatory support time.46
Recommendations
-
PLEX and IVIg are indicated in (1) MG crisis (respiratory insufficiency), or severe exacerbation; (2) when a patient with significant bulbar or respiratory dysfunction is being prepared for an emergent surgery; (3) maintenance therapy for the refractory forms, or when corticosteroids are contraindicated (such as in the patients with uncontrollable diabetes mellitus).
-
Choosing between PLEX and IVIg should be individualized according to each center’s experience, availability, cost, and the patient’s condition – e.g. in patients with a history of cardio-embolic events or renal failure, PLEX may be preferable; however, in patients with sepsis, IVIg may be superior to PLEX. With PLEX, the risk of hemodynamic instability and venous access problems is always a concern.
Thymectomy
There is general agreement on thymectomy in thymomatous-MG2,3; however, for decades, there have been controversies about non-thymomatous patients due to the lack of randomized controlled trials.50-52 In a multi-center, randomized, rater-blinded clinical trial, 126 AChR + generalized MG patients, aged 18-65 years, were randomized to either thymectomy plus prednisone versus prednisone alone. The thymectomy arm had a lower score in average QMG and lower prednisone dose requirement in a 3-year follow-up.53 The extension study evaluated the outcome after five years of follow-up. Thymectomy plus prednisone continued to confer benefits in generalized non-thymomatous AChR + MG patients compared with prednisone alone.54
CT scan with intravenous contrast is the imaging modality of choice for assessing thymus abnormalities.2,55 Thymectomy for MG is an elective surgery; however, it must be done as soon as possible in thymomatous patients, and it should be done when the patient is in a stable condition. The benefit of thymectomy is not immediate and appears in the following 1–2 years.56 The current belief is that the benefits of thymectomy are most significant if performed within the first three years of initial symptoms.57,58 One study in Iran showed that early thymectomy (less than one year after MG diagnosis) is beneficial.59 Four operative procedures are used for thymectomy: (1) Transcervical; (2) minimally invasive (video-assisted [VATS] and robot-assisted); (3) transsternal; (4) mixed transcervical-transsternal. VATS and robotic methods seem to profit comparable outcomes to more aggressive techniques, even in MG with thymoma.60,61 However, it is unknown whether the benefit achieved by extended transsternal thymectomy can also be achieved by minimally invasive approaches such as VATS.51
Recommendations
-
Chest CT with contrast must be done in all MG patients with stable conditions. Chest MRI should be considered if the contrast injection is contraindicated. Whether MuSK + MG patients require chest imaging is not clear
-
Thymectomy is recommended for generalized non-thymomatous AchR + MG patients aged 18 through 65 and all thymomatous MG patients (Figure 1).
-
In juvenile MG, thymectomy decisions should be individualized based on the patient’s clinical condition and VATS availability.
-
There is no consensus on patients with generalized MG with double seronegative antibody status.
-
Thymectomy is not indicated in MuSK + MG patients.
-
Thymectomy is not recommended in patients with ocular MG unless there is evidence of thymoma on chest CT scan.
-
In patients with the myasthenic crisis or patients with severe weakness and bulbar dysfunction, the operation must be performed after stabilization (using PLEX, IVIg, or other medications)
-
It is not clear whether less-invasive methods such as VATS are as effective as trans-sternal thymectomy
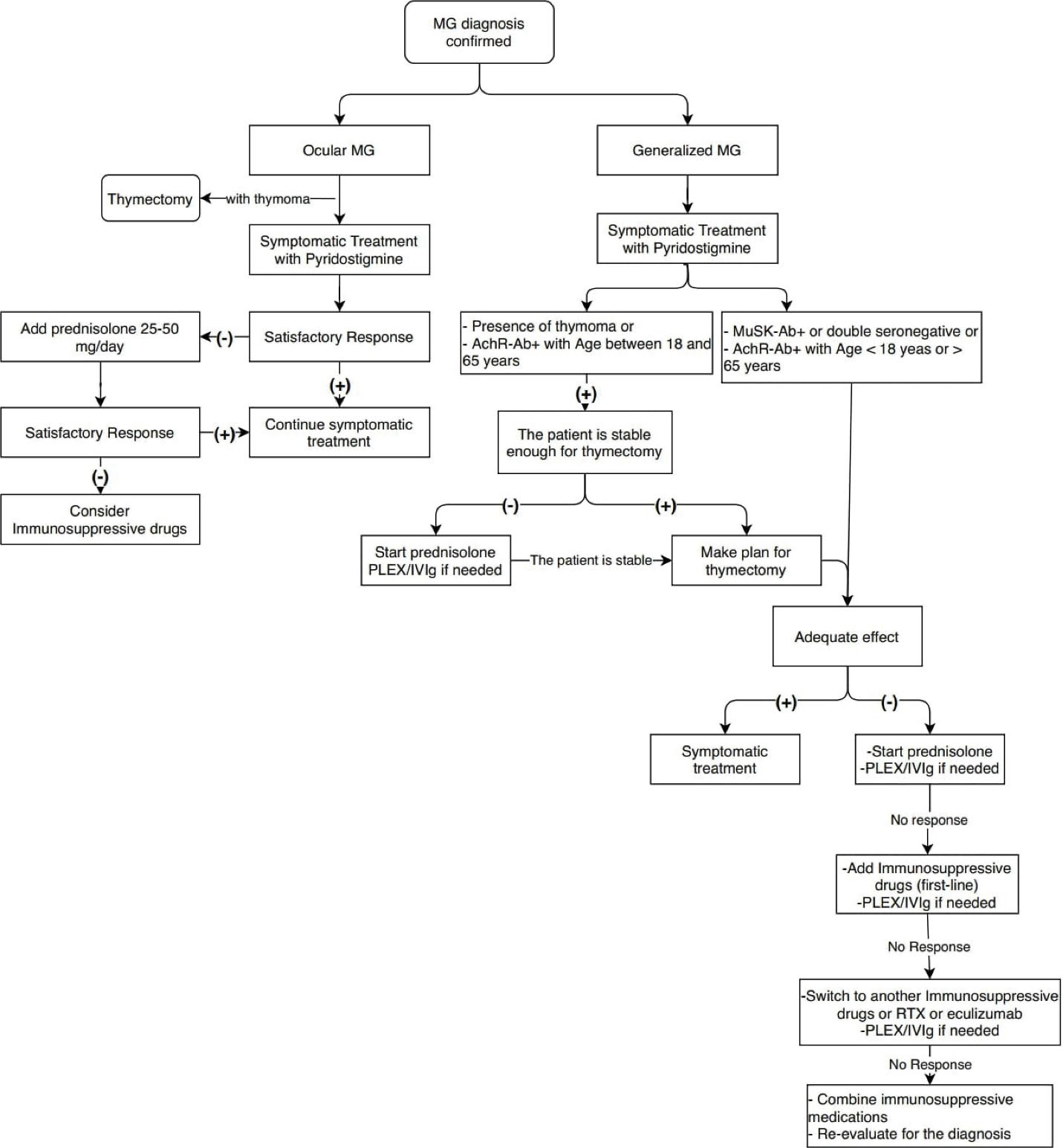
Figure 1.
Algorithm of MG treatment.
.
Algorithm of MG treatment.
Immunosuppressive Therapy in Management of MG
Corticosteroids
Like other autoimmune diseases, immunosuppressive therapy is the mainstay of MG treatment, most notably with corticosteroids (Algorithm 1). Although the number of double-blind, placebo-controlled studies providing credible clinical evidence about the effectiveness of corticosteroids in MG is limited, many retrospective studies have shown its effectiveness in alleviating symptoms in more than 80% of myasthenic patients.2,62
Several corticosteroid regimens have been recommended for MG. One of the most frequently used regimens is high-dose prednisolone (1–1.5 mg/kg/d). In this regimen, clinical improvement is expected within 2-4 weeks, although complete remission might not ensue until several months later. After observing a prompt clinical response, the corticosteroid dose would be lowered gradually, at a rate of 5–10 mg/mon.1,62 The primary concern with the high dose regimen is clinical deterioration in the first few days of the treatment. Therefore, the protocol could only be administered in an inpatient setting.
The second approach involves low-dose (5–10 mg/d) corticosteroids administration with subsequent escalation by 5–10 mg weekly until the symptoms are resolved or the maximum dose of 1–1.5 mg/kg/d reached. Corticosteroids should be tapered upon the induction of the clinical response. The main benefit of this approach is the lower occurrence of the above-mentioned initial deterioration, though at the expense of delayed remission.1
Some experts have recently pursued a third approach: a medium dose of prednisone (e.g. 20 mg/d) is administered first. Further escalation will be considered if clinical remission is not evident at monthly clinical evaluations. Like the two approaches mentioned above, corticosteroids should be tapered as soon as induction is achieved.2
As a fourth approach, corticosteroid pulse therapy has been used to induce remission in myasthenic patients. However, clinical studies are scarce, so pulse therapy has not gained much attention as a treatment choice in MG.63
In patients with ocular or mild forms of MG, corticosteroids are used as the sole immunosuppressant.2 However, most MG patients, especially those with moderate and severe disease, will need higher corticosteroid doses for long periods. Adverse effects might outweigh the benefits in this situation1,2 and addition of immunosuppressive drugs is reasonable.
Non-steroidal Immunosuppressive Drugs
The efficacy of azathioprine (AZT), mycophenolate mofetil, methotrexate, tacrolimus, and cyclophosphamide has been investigated in double-blind placebo-controlled clinical trials.
AZT is the most acceptable steroid-sparing agent in MG treatment.64 In a double-blind randomized trial of 34 patients, the prednisolone dose and clinical outcome were assessed over a follow-up period of 3 years in patients taking prednisolone and AZT and those taking prednisolone and placebo. The placebo group was more prone to relapses and failure to remit. 64 Delayed clinical response (until at least one year) is believed to be the primary concern with the use of AZT and the need for periodic liver function and CBC monitoring.2
Mycophenolate mofetil is another potentially effective immunosuppressive with the main advantage of a favorable safety profile leading to high compliance.1,2 This medication is found to be potentially effective by some neuromuscular experts, although it has failed to yield significant results in 2 clinical trials65,66; however, trial failure may be due to a short follow-up period (36 weeks in one study and 12 weeks in the other) and simultaneous use of prednisolone. Nevertheless, its favorable safety profile leading to high compliance makes it a good alternative steroid-sparing option.
The methotrexate trial also showed non-significant results with a dose of 20 mg weekly over 12 months.67 The only trial with tacrolimus also showed no significant difference between tacrolimus and placebo group over 28 weeks, although in secondary analyses, some steroid-sparing effects of tacrolimus were suggested.68
Monthly intravenous cyclophosphamide has been investigated in 23 MG patients with poor disease control or corticosteroid-related side effects for 12 months. Favorable results were achieved in muscle force, steroid and pyridostigmine dose reductions, and bulbar muscle weakness in the cyclophosphamide group.69
Several clinical trials have been performed on cyclosporine between 1987 and 2010, and most of them have suggested the efficacy of cyclosporine in MG; however, no double-blind placebo-controlled trial could be found.70,71
Monoclonal Antibodies and Emerging Therapies
Rituximab is a newly introduced promising option in MG treatment.4-6 The efficacy of RTX in MuSK + MG and AchR + MG is well established.72,73 Availability, relative safety, and ease of administration of rituximab have led to a significant drop in cyclophosphamide use in MG. Currently, there is no consensus on the inclusion of rituximab as a therapeutic choice in MG. However, treatment of resistant disease is probably the main indication for rituximab in MG.1,2
Ocrelizumab is a new generation fully humanized anti-CD20 monoclonal antibody, which probably has the same effects as rituximab.74 After a successful clinical trial of eculizumab (a monoclonal complement inhibitor antibody), FDA has approved Soliris for refractory generalized AchR + MG.38 This drug is not available in Iran yet. Ravulizumab, which binds the complement protein C5like eculizumab, is under study in phase III clinical trial. Ravulizumab has a longer half-life than eculizumab.76
Zilucoplan is a subcutaneously-administered C5 inhibitor peptide, and the results of phase 2 trials on Zilucoplan have been favorable in reducing QMG and MG-ADL.77 Efgartigimod, the neonatal Fc-receptor (FcRn) antagonist, shortens IgG half-life.78 In phase II clinical trial, all MG patients treated with efgartigimod exhibited fast reduction in overall IgG and anti-AChR autoantibody levels, and 75% of the patients showed fast and long-lasting disease control.78
Ongoing Monitoring in MG Treatment
Laboratory test monitoring during MG treatment differs based on the type of immunosuppressant treatment. Bone mineral densitometry must be performed in the first six months of treatment for all patients above 40 years of age and any patients with other osteoporosis risk factors. Repeat bone mineral densitometry should be performed every 1-3 years during treatment.
Preceding the start of treatment, one must consider if vaccinations are needed and laboratory tests should be evaluated. Moreover, some laboratory tests are specific to each distinct immunosuppressant with regular testing79:
AZT; CBC with differential, creatinine, liver function tests (LFTs), Mycophenolate mofetil; CBC with differential, Methotrexate; CBC with differential, creatinine, LFTs. Rituximab; CBC with differential and immunoglobulins before each cycle, hepatitis B PCR every 6 months if core antibody (HBc Ab) is positive, Cyclosporine; CBC with differential, creatinine, LFTs, lipid panel, Cyclophosphamide; CBC with differential and creatinine.79
Recommendations
-
In mild generalized AchR + MG, start treatment with ChEIs plus thymectomy if indicated. Add prednisolone if symptoms persist or exacerbate after thymectomy (Algorithm 1).
-
In moderate-severe generalized AchR + MG, start treatment with ChEIs plus prednisolone as well as thymectomy if indicated.
-
Add AZT to corticosteroids when: (1) significant steroid side effects occur; (2) response to an acceptable corticosteroid dose is insufficient; (3) the patient is steroid-dependent (relapse of symptoms with reduction of corticosteroid dose) with a dose so high that long-term maintenance is not acceptable.
-
If AZT or other immunosuppressives such as mycophenolate mofetil, cyclosporine, or tacrolimus are ineffective (after 9-12 months of treatment), replace with rituximab.
-
In moderate-severe generalized AchR + MG, when waiting for the effect of immunosuppressives, treat exacerbations with intermittent IVIg or PLEX.
-
If the patient is refractory to previous agents, consider monthly IVIg; if non-responsive, consider cyclophosphamide, or eculizumab (in non-thymomatous generalized AchR + MG).
-
In patients who are contraindicated for corticosteroids, it is possible to start immunosuppressive as an initial immunosuppressive agent.
-
After treatment with PLEX/IVIg, in MG crisis, consider augmentation of long-term immunosuppression with prednisone or other immunosuppressives if necessary.
MG Treatment in Specific Settings
Ocular MG
ChEIs alone usually will not resolve the ocular symptoms. Most guidelines recommend oral corticosteroids if symptoms are functionally restricting or worrying to the patient.80,81 A randomized, double-blind trial (EPITOME) has reported good results for oral prednisolone in the improvement of ocular symptoms.82 A inferior dose than in generalized MG (25–30 mg/d) can be often effective.80,81 Almost one-third of the patients need treatment for a long-term period.
Recommendations
-
ChEIs are recommended as the initial treatment for ocular MG.
-
We recommend corticosteroids at a dose of 25-30 mg/d in ocular MG unresponsive to ChEIs and increasing the dose to 1 mg/kg if the response is not optimal. As soon as the optimal response is achieved, tapering of corticosteroids should be initiated slowly (e.g. 2.5 to 5 mg monthly) to the lowest tolerable dose.
-
In case of significant residual symptoms, corticosteroid-sparing immunosuppressive drugs could be required after corticosteroids alone are unsuccessful, contraindicated, or not tolerated.
Anti-MuSK and LRP4 Positive MG
MuSK MG is a subgroup of the disease, accounting for less than 10% of all MG patients. The dominance of cranial, bulbar, and axial muscle involvement and the higher likelihood of early respiratory failure give rise to an aggressive picture that mandates consequent aggressive treatments.83 Some MuSK + MG patients do not respond to pyridostigmine.84 Standard doses of pyridostigmine frequently bring noticeable nicotinic side effects in these subjects, apparent both clinically (diffuse fasciculations and cramps) and at electrodiagnosis.21
Currently, based on pathological and clinical data, thymectomy is not recommended in anti-MuSK MG.85,86
Studies show that MuSK + MG patients respond well to steroids and dramatically to PLEX.83 The great majority of patients (95 to 100% in different series) need immunosuppression therapy.87 In a study by Evoli et al on 57 MuSK + patients, most patients (> 90%) needed immunosuppressive treatment, and 35 patients were treated with one or more courses of PLEX and IVIg. The complete remission rate was less than 10%, and immunosuppressive treatment had been withdrawn in only less than 20% of the patients.88 Rituximab may be a promising medication for Anti-MuSK + MG treatment. In a recent study on anti-MuSK + patients, 30% of the rituximab-treated MG patients used prednisone (mean dose 4.5 mg/day) compared to 75% of the controls (mean dose 13 mg/d).89
LRP4, a receptor for agrin and an activator of MuSK, is expressed in the postsynaptic muscle membrane.90 LRP4 antibodies have been seen in 2–30% of the MG patients without AChR and MUSK antibodies (double seronegative), with a female dominance. Most of the patients manifest with ocular or generalized mild MG (20% of LRP4 + patients with ocular MG), and respiratory insufficiency is infrequent in these cases.90 Usually, anti-LRP4 patients have a mild phenotype, and their treatment mostly resembles AchR-Ab patients.90
Recommendations
-
ChEIs can be used as the symptomatic treatment of patients with MuSK + MG; however, their efficacy may be less than AchR-Ab MGs with a greater chance of side effects and intolerability. Start AchEIs in MuSK patients at lower doses, and warn the patient of the possible side effects.
-
For MuSK + MG patients with exacerbation or crisis, PLEX is preferred over IVIg.
-
Most MuSK + MG patients required chronic corticosteroids treatment and have a favorable response to corticosteroids, but there is usually a recurrence of symptoms after tapering.
-
Many MuSK + MG patients require immunosuppressives treatment as an add-on to corticosteroids: the immunosuppressive therapy of choice is rituximab. The exception is patients with mild symptoms and the subgroup that are effectively controlled by low dose corticosteroids.
Thymoma and Myasthenia Gravis
Thymoma is the most common mediastinal tumor among adults. This tumor mostly occurs between 50 and 65 years of age, and there is no meaningful difference between the two sexes.91 The occurrence of MG in thymoma patients is about 50%, and 15% of MG patients have thymoma.92 AchR autoantibody is almost always positive in thymoma patients93 supporting the notion that MG is a paraneoplastic manifestation of thymoma.93 MG is more often related to WHO type B thymoma,94 and it does not usually occur in thymus carcinoma, which does not have a thymic epithelial origin.95 Ryanodine receptor antibodies are present in 95% of thymomatous-MG patients and are associated with a more aggressive course.92 Respiratory symptoms are more prominent in Titin antibody-positive patients than other patients.96
Like non-myasthenic thymoma, thymectomy, radiotherapy, and chemotherapy are the mainstay of treatment in myasthenic patients. Radical resection is the standard treatment in most cases of thymoma. Different approaches, including median sternotomy and VATS, have been used for thymectomy in thymoma patients. Although current evidence comes from retrospective studies, VATS is considered a safe approach in skilled centers. In 2011, Zahid et al published a review of 74 papers on comparing VATS with median sternotomy. They concluded that VATS had superior results in the length of hospital stay, perioperative blood loss, and patient’s satisfaction.97 In 2018, Ersen and colleagues reported no substantial difference in perioperative blood loss and surgery duration or pain between VATS and median sternotomy. However, the chest tube drainage period after surgery and hospitalization length were shorter in the VATS group.98
Radiotherapy ± chemotherapy is also considered in stage 2 thymomas with a high risk of recurrence and stage 3 and 4 of the tumor when radical resection of thymoma is not possible.99,100 In 2011, Romi published a review article on the management of thymoma in MG. They emphasized that other pharmacological treatments like acetylcholinesterase inhibitors, steroids, and steroid-sparing drugs are used in thymoma MG, like non-thymoma, for controlling myasthenic symptoms.92
Recommendations
-
All patients with thymoma, except stage 4b, who have diffuse metastasis, should undergo thymectomy regardless of MG status if there are no other contraindications.
-
There is no significant difference between VATS and medial sternotomy, although because patients tolerate VATS better than open surgery, we recommend VATS, where there is enough expertise.
-
Management of myasthenia symptoms and maintenance therapy do not vary between thymomatous and non-thymomatous MG patients. However, thymomatous MG patients have a more severe disease course and higher chance of relapse and probably more frequent and prolonged use of immunosuppressives.
-
We suggest evaluating the recurrence of tumors in refractory patients or cases of relapse.
-
Follow-up chest CT scan 6–12 months after surgery is recommended.
MG in Elderly Patients (Late-Onset MG)
In a large series of late-onset MG, 837 MG patients were followed for 22 years, including 172 late-onset MG.101 In 67 patients treated with prednisone, improvement was observed in 76% patients and severe side effects in 18%. In 46 MG patients who received treatment with prednisone plus AZT, improvement was observed in 89% and severe side effects in 19.5%. In seven late-onset MG patients treated with AZT alone, improvement was recorded in five with no side effects.101
Recommendations
-
In late-onset, generalized AchR + MG (> 65 years), start treatment with ChEIs plus AZT with or without prednisolone.
-
Thymectomy is not recommended unless thymoma is suspected
Pregnancy Issues in MG
MG crisis or exacerbation occurs mainly in the 1st trimester of pregnancy and postpartum period.102 Several studies have shown that pyridostigmine does not cross the placenta, and there have been no associations with fetal anomalies. The American Academy of Pediatrics considers pyridostigmine compatible with breastfeeding.103 However, intravenous AChEIs may produce uterine contractions and should not be used during pregnancy.104
Maintenance corticosteroids on the lowermost feasible dose can be maintained in pregnancy,103 and prednisolone is the preferred corticosteroid in pregnancy.104 Patients receiving more than 7.5 mg/d prednisolone for more than two weeks before delivery must obtain parenteral corticosteroids for stress dose.105 In contrast to prednisolone, methylprednisolone is accompanied by an increased risk of cleft palate once used in the 1st trimester.105 Corticosteroids can be continued during breastfeeding. Some experts recommend discarding the breast milk for the first four hours after consumption of prednisolone dose more than 20 mg.102
Regarding immunosuppressants,AZT has not been related to increased rates of congenital abnormalities.106 Therefore, it seems that AZT is harmless to administer during pregnancy and breastfeeding, but the drug dose should be below 100 mg/d when possible.107
Mycophenolate mofetil has had teratogenic effects in human studies. It is related to 1st trimester abortion, and other structural anomalies.108
Methotrexate is contraindicated in pregnancy and breastfeeding. In women who plan for pregnancy and are using methotrexate, a wash-out period of at least three months should be considered before pregnancy.105
Cyclosporine and tacrolimus are not teratogenic. However, the risk of gestational diabetes mellitus and hypertension increases with tacrolimus use in pregnancy.109,110
IVIg and PLEX are considered to be safe in the treatment of acute myasthenic exacerbation during pregnancy.102
According to several studies, vaginal delivery is suggested for MG pregnant women.111 Cesarean section must be done only for obstetric indications. Some studies have suggested avoding general anesthesia and narcotics as they can synergistically potentiate AChR antibody effects.112
Recommendations
-
Oral ChEIs are the first choice of treatment for MG during pregnancy, and they are safe.
-
Prednisolone is safe during pregnancy as the first choice of immunomodulation drug with the lowest effective dose.
-
Mycophenolate mofetil, cyclophosphamide, and methotrexate are contraindicated during pregnancy.
-
If immunosuppressive therapy is necessary for pregnancy, AZT and cyclosporine are relatively safe agents that could be continued during pregnancy with the lowest effective dose.
-
During a crisis, IVIg, or PLEX, could be used in pregnancy considering the mother and fetus’s risk-benefit.
-
Vaginal delivery is suggested for pregnant MG women as the first choice, and a Cesarean section must be carried out only for obstetric indications.
Treatment of MG in COVID Era
The fatality rate of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in hospitalized adult patients is 2%–3%. However, this rate can rise to 11% in the elderly and those with underlying comorbidities and immunocompromised states such as MG.113,114
Currently, there are limited data on how COVID-19 infects people with MG. Theoretically, there is a higher risk of suffering severe manifestations of COVID-19 owing to the frequent use of immunosuppressive drugs and potentially oropharyngeal and respiratory weakness.115,116
Therapy choices must be modified and made interactively between the patient with MG and physicians.
Recommendations
-
MG patients must remain their treatment and are recommended not to stop any existing medications unless accepted by their physician.
-
We recommend that MG patients even now on immunosuppressive treatments must practice face masking, extra-attentive social distancing, as well as avoiding public meetings and overcrowded public transportation. Also, frequent hand-washing and use of alcohol-based hand antiseptics should be considered.
-
We recommend continuing symptomatic treatment with pyridostigmine as before.
-
Although IVIg and plasmapheresis probably do not increase the risk of contracting the virus, reducing the frequency of infusions in stable patients and home infusions should be considered.
-
In the case of corticosteroid use, consider the following: Prescribe the minimum required dose that prevents flare-ups, do not stop the drug abruptly, give the stress dose in the case of acute illness.
-
For immunosuppressive drugs such as AZT, mycophenolate mofetil, cyclosporine, etc., it is recommended to continue treatment in patients on treatment with these drugs. If clinically plausible, delay the administration of new immunosuppressive until the epidemic is over.
-
For B-cell depleting therapies such as rituximab, consider alternative treatments if deciding which IS to start, injections at longer intervals (especially if CD19 counts are lower than 0.5%), or in smaller amounts.
Transient Neonatal MG (TNMG)
Ten to 30% of neonates born to mothers with MG develop TNMG. Infants with TNMG typically show weak cry, poor sucking, or ptosis 12 hours to four days after birth that continues from two weeks to several months afterwards.117 If the symptoms are transient and mild, no treatment is usually required. If symptoms are troublesome, pyridostigmine should be considered.118 One study recommended early treatment with PLEX once hydramnios or swallowing weakness in the newborn appear.119
Recommendations
-
All infants with TNMG should be observed and examined for evidence of transient myasthenia weakness mainly through the first week and should have rapid access to neonatal critical care support (NICU).
-
If symptoms are transient and mild, wait and observe or try pyridostigmine (Oral: 5 mg each 4 to 6 hours IM, IV: 0.05 to 0.15 mg/kg/dose).
-
In severe cases, start IVIg/PLEX and mechanical ventilation when necessary.
-
Once hydramnios or weakened swallowing in the newborn appear, it is required to treat early with IVIg/PLEX.
-
Corticosteroids and immunosuppressive are not indicated for TNMG.
Vaccination
Several placebo-controlled and retrospective studies have assessed the safety and efficacy of different vaccines in MG patients. Generally, inactivated vaccines are considered safe in MG patients. A prospective survey of tetanus revaccination showed a lower but significant immune response with no increased MG exacerbation risk.120 The humoral immune response to diphtheria and tetanus vaccinations was comparable with healthy controls in a retrospective investigation.121
Influenza vaccination has proven to be safe in retrospective studies, and the only randomized clinical trial of the Influenza vaccine in MG patients also approved its safety.122 Furthermore, it has been found that the risk of aggravating MG after an influenza-like illness was more than the risk associated with influenza vaccination, supporting routine influenza vaccination in MG patients.123 Live-attenuated vaccines must be avoided in MG patients on immunosuppressive treatment. Preferably, these vaccines should be considered 2 to 4 weeks before immunosuppressive therapy initiation 124. In the case of rituximab planning, vaccination must be programmed before initiating the therapy or postponed to at least six months after the drug’s latest cycle.124
Recommendations
-
Inactivated vaccines can be used in the same way as the general population vaccination program.
-
Live-attenuated vaccines must be avoided in MG patients on immunosuppressive therapy or planned to be used 2-4 weeks before immunosuppressive initiation.
-
Vaccination must be programmed before initiating rituximab or postponed to at least six months after the drug’s latest cycle.
Drugs to Avoid in MG
Different lists of drugs have been proposed to be contraindicated in MG according to case reports, in vitro studies, or retrospective data.125 Two categories of drugs seem to be more critical. The first is drugs and conditions known to induce autoimmune MG, including D-penicillamine, interferon-alpha and beta, etanercept, imiquimod, tandutinib, and immune checkpoint inhibitors. The other group is medications with black box warnings of FDA, namely botulinum toxin,125 fluoroquinolones (e.g., ciprofloxacin and levofloxacin), and telithromycin (i.e., Ketek). Other potentially aggravating drugs include125: other antibiotics: aminoglycosides (such as gentamycin, neomycin), azithromycin, tetracyclines, sulfonamides, amino acid antibiotics (polymyxin B), nitrofurantoin, clindamycin and lincomycin, colistin, colistimethate; cardiovascular drugs, such as verapamil, procainamide, bretylium, quinidine, quinine, and oral magnesium; antiepileptic drugs, such as phenytoin and barbiturate; neuromuscular blocking agents and local anesthetics; eye drugs: timolol, betaxolol; psychiatric drugs: lithium; iodinated contrast.126
Recommendations
-
Drugs that induce MG, those with black box warnings including injectable magnesium, immune checkpoint inhibitors, and botulinum toxin, should be avoided in MG patients unless in life-threatening conditions when no alternative exists.
-
Other drugs that need particular caution include: fluoroquinolones (e.g., ciprofloxacin and levofloxacin), telithromycin (i.e., Ketek), aminoglycosides (such as gentamycin, neomycin), azithromycin, tetracyclines, sulfonamides, amino acid antibiotics (polymyxin B), nitrofurantoin, clindamycin, and lincomycin, colistin, verapamil, procainamide, bretylium, quinidine, quinine, oral magnesium, phenytoin and barbiturate, neuromuscular blocking agents and local anesthetics, eye drop (timolol, betaxolol), lithium, iodinated contrast
-
Regarding other drugs that can potentially worsen MG, clinical judgment is necessary not to deprive patients of receiving appropriate treatments.
Perioperative Considerations
Two retrospective studies have been conducted on MG patients preoperatively. One has compared routine preoperative PLEX with selective PLEX of severe cases.127 The other has evaluated PLEX versus no additional treatment in stable patients.128 None showed a significant change between the two groups. There is no international or regional consensus for perioperative management of MG patients to date. The only related studies are those evaluating the likelihood of a post-surgical myasthenic crisis. In one study, it was found that patients with a duration of disease more than six years, preceding respiratory complaints, pyridostigmine doses more than 750 mg/d, and forced vital capacity less than 2.9 L preoperatively were associated with postoperative respiratory failure.129–131
Recommendations
-
Routine PLEX/IVIg is not indicated in all MG patients preoperatively. PLEX is preferred in MG patients with moderate to severe manifestations before surgery due to its faster onset of action.
-
Pay special attention to the severity of the disease, mainly respiratory function and bulbar symptoms.
-
The best time to perform an elective surgery is when the patient is in stable conditions.
-
In emergency surgeries, plasmapheresis should be considered in an MG patient with moderate to severe manifestations.
-
Steroid stress dose is similar to other patients taking chronic corticosteroids.
-
Routine pre-treatment with sedative or opioid medications should be prohibited as they increase the risk of respiratory depression.
-
Depolarizing neuromuscular blocking agents are preferred over non-depolarizing drugs. If non-depolarizing agents must be used, short- and intermediate-acting agents are prioritized.
-
Although there is a theoretical increase in the risk of vagal responses and the dose of non-depolarizing neuromuscular blocking agents by AChEIs, it is recommended to keep the same dose of AChEIs in stable MG patients perioperatively.
-
Although AZT and corticosteroids may interfere with some anesthetic drugs, theoretically, there is no need to change the dose of corticosteroids and immunosuppressives in a stable patient.
In conclusion, in this guideline, we attempted to develop a guideline based on Iran’s local requirements. We hope that these guidelines help healthcare professionals with proper treatment and follow-up of patients with MG.
Authors’ Contribution
SN and FF organized the panel sessions. FF, AAO, FS, BA, HA, KB, RB, BHA, PS, and SN contributed to consensus preparation, including literature review and writing the initial draft for each section, and also the final manuscript. All authors have read and approved the final manuscript.
Conflict of Interest Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Statement
The study was conducted as per the ethical principles of the Helsinki Declaration.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
References
- Ciafaloni E. Myasthenia gravis and congenital myasthenic syndromes. Continuum (Minneap Minn) 2019; 25(6):1767-84. doi: 10.1212/con.0000000000000800 [Crossref] [ Google Scholar]
- Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ. Treatment of myasthenia gravis. Neurol Clin 2018; 36(2):311-37. doi: 10.1016/j.ncl.2018.01.011 [Crossref] [ Google Scholar]
- Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016; 87(4):419-25. doi: 10.1212/wnl.0000000000002790 [Crossref] [ Google Scholar]
- Anderson D, Phan C, Johnston WS, Siddiqi ZA. Rituximab in refractory myasthenia gravis: a prospective, open-label study with long-term follow-up. Ann Clin Transl Neurol 2016; 3(7):552-5. doi: 10.1002/acn3.314 [Crossref] [ Google Scholar]
- Beecher G, Anderson D, Siddiqi ZA. Rituximab in refractory myasthenia gravis: Extended prospective study results. Muscle Nerve 2018; 58(3):452-5. doi: 10.1002/mus.26156 [Crossref] [ Google Scholar]
- Marino M, Basile U, Spagni G, Napodano C, Iorio R, Gulli F. Long-lasting rituximab-induced reduction of specific-but not total-IgG4 in MuSK-positive myasthenia gravis. Front Immunol 2020; 11:613. doi: 10.3389/fimmu.2020.00613 [Crossref] [ Google Scholar]
- Howard JF Jr. Electrodiagnosis of disorders of neuromuscular transmission. Phys Med Rehabil Clin N Am 2013; 24(1):169-92. doi: 10.1016/j.pmr.2012.08.013 [Crossref] [ Google Scholar]
- Sanders DB. Clinical impact of single-fiber electromyography. Muscle Nerve Suppl 2002; 11:S15-20. doi: 10.1002/mus.10141 [Crossref] [ Google Scholar]
- Keesey JC. Clinical evaluation and management of myasthenia gravis. Muscle Nerve 2004; 29(4):484-505. doi: 10.1002/mus.20030 [Crossref] [ Google Scholar]
- Evoli A, Padua L. Diagnosis and therapy of myasthenia gravis with antibodies to muscle-specific kinase. Autoimmun Rev 2013; 12(9):931-5. doi: 10.1016/j.autrev.2013.03.004 [Crossref] [ Google Scholar]
- Oh SJ, Cho HK. Edrophonium responsiveness not necessarily diagnostic of myasthenia gravis. Muscle Nerve 1990; 13(3):187-91. doi: 10.1002/mus.880130302 [Crossref] [ Google Scholar]
- Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord 2006; 16(7):459-67. doi: 10.1016/j.nmd.2006.05.006 [Crossref] [ Google Scholar]
- Mehanna R, Patton EL Jr, Phan CL, Harati Y. Amyotrophic lateral sclerosis with positive anti-acetylcholine receptor antibodies. Case report and review of the literature. J Clin Neuromuscul Dis 2012; 14(2):82-5. doi: 10.1097/CND.0b013e31824db163 [Crossref] [ Google Scholar]
- Andreetta F, Rinaldi E, Bartoccioni E, Riviera AP, Bazzigaluppi E, Fazio R. Diagnostics of myasthenic syndromes: detection of anti-AChR and anti-MuSK antibodies. Neurol Sci 2017; 38(Suppl 2):253-7. doi: 10.1007/s10072-017-3026-2 [Crossref] [ Google Scholar]
- Zisimopoulou P, Evangelakou P, Tzartos J, Lazaridis K, Zouvelou V, Mantegazza R. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J Autoimmun 2014; 52:139-45. doi: 10.1016/j.jaut.2013.12.004 [Crossref] [ Google Scholar]
- Evoli A, Antonini G, Antozzi C, DiMuzio A, Habetswallner F, Iani C. Italian recommendations for the diagnosis and treatment of myasthenia gravis. Neurol Sci 2019; 40(6):1111-24. doi: 10.1007/s10072-019-03746-1 [Crossref] [ Google Scholar]
- Mehndiratta MM, Pandey S, Kuntzer T. Acetylcholinesterase inhibitor treatment for myasthenia gravis. Cochrane Database Syst Rev 2014; 2014(10):CD006986. doi: 10.1002/14651858.CD006986.pub3 [Crossref] [ Google Scholar]
- Ricciardi R, Rossi B, Nicora M, Sghirlanzoni A, Muratorio A. Acute treatment of myasthenia gravis with intranasal neostigmine: clinical and electromyographic evaluation. J Neurol Neurosurg Psychiatry 1991; 54(12):1061-2. doi: 10.1136/jnnp.54.12.1061 [Crossref] [ Google Scholar]
- Ionita CM, Acsadi G. Management of juvenile myasthenia gravis. Pediatr Neurol 2013; 48(2):95-104. doi: 10.1016/j.pediatrneurol.2012.07.008 [Crossref] [ Google Scholar]
- Chiang LM, Darras BT, Kang PB. Juvenile myasthenia gravis. Muscle Nerve 2009; 39(4):423-31. doi: 10.1002/mus.21195 [Crossref] [ Google Scholar]
- Oh SJ. Muscle-specific receptor tyrosine kinase antibody positive myasthenia gravis current status. J Clin Neurol 2009; 5(2):53-64. doi: 10.3988/jcn.2009.5.2.53 [Crossref] [ Google Scholar]
- Gajdos P, Chevret S, Toyka K. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev 2006; 2(#):CD002277. doi: 10.1002/14651858.CD002277.pub2 [Crossref] [ Google Scholar]
- Jongen JL, van Doorn PA, van der Meché FG. High-dose intravenous immunoglobulin therapy for myasthenia gravis. J Neurol 1998; 245(1):26-31. doi: 10.1007/s004150050170 [Crossref] [ Google Scholar]
- Ferrero B, Durelli L. High-dose intravenous immunoglobulin G treatment of myasthenia gravis. Neurol Sci 2002; 23 Suppl 1:S9-24. doi: 10.1007/s100720200011 [Crossref] [ Google Scholar]
- Wegner B, Ahmed I. Intravenous immunoglobulin monotherapy in long-term treatment of myasthenia gravis. Clin Neurol Neurosurg 2002; 105(1):3-8. doi: 10.1016/s0303-8467(02)00017-3 [Crossref] [ Google Scholar]
- Gajdos P, Chevret S, Toyka K. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev 2003; 2(#):CD002277. doi: 10.1002/14651858.cd002277 [Crossref] [ Google Scholar]
- Arsura EL, Bick A, Brunner NG, Namba T, Grob D. High-dose intravenous immunoglobulin in the management of myasthenia gravis. Arch Intern Med 1986; 146(7):1365-8. doi: 10.1001/archinte.1986.00360190143020 [Crossref] [ Google Scholar]
- Bonaventura I, Ponseti J, Arnau E, Matias-Guiu J, Codina Puiggros A. High-dose intravenous immunoglobulin in the management of myasthenia gravis. Arch Intern Med 1987; 147(2):207-11. doi: 10.1001/archinte.1987.00370020027019 [Crossref] [ Google Scholar]
- Evoli A, Palmisani MT, Bartoccioni E, Padua L, Tonali P. High-dose intravenous immunoglobulin in myasthenia gravis. Ital J Neurol Sci 1993; 14(3):233-7. doi: 10.1007/bf02335664 [Crossref] [ Google Scholar]
- Kumar R, Birinder SP, Gupta S, Singh G, Kaur A. Therapeutic plasma exchange in the treatment of myasthenia gravis. Indian J Crit Care Med 2015; 19(1):9-13. doi: 10.4103/0972-5229.148631 [Crossref] [ Google Scholar]
- Taiuti R, Avanzi G, Paoletti P, Marconi G. Plasma-exchange in myasthenia gravis: a study in 20 patients. Int J Artif Organs 1988; 11(4):308-12. [ Google Scholar]
- Fornádi L, Horváth R, Szobor A. Myasthenia gravis: treatment with plasma exchange experiences over 10 years. Acta Med Hung 1991; 48(3-4):137-44. [ Google Scholar]
- Kuks JB, Das PC. Plasma exchange in myasthenia gravis. Int J Artif Organs 1998; 21(4):188-91. [ Google Scholar]
- Gajdos P, Chevret S, Toyka K. Plasma exchange for myasthenia gravis. Cochrane Database Syst Rev 2004; 4(#):CD002275. doi: 10.1002/14651858.cd002275 [Crossref] [ Google Scholar]
- Durelli L, Cocito D, Bergamini L. Rapid improvement of myasthenia gravis after plasma exchange?. Ann Neurol 1983; 13(2):220-1. doi: 10.1002/ana.410130231 [Crossref] [ Google Scholar]
- d’Empaire G, Hoaglin DC, Perlo VP, Pontoppidan H. Effect of prethymectomy plasma exchange on postoperative respiratory function in myasthenia gravis. J Thorac Cardiovasc Surg 1985; 89(4):592-6. [ Google Scholar]
- Thorlacius S, Aarli JA, Jacobsen H, Halvorsen K. Plasma exchange in myasthenia gravis: clinical effect. Acta Neurol Scand 1985; 72(5):464-8. doi: 10.1111/j.1600-0404.1985.tb00902.x [Crossref] [ Google Scholar]
- Keesey J, Buffkin D, Kebo D, Ho W, Herrmann C, Jr Jr. Plasma exchange alone as therapy for myasthenia gravis. Ann N Y Acad Sci 1981; 377:729-43. doi: 10.1111/j.1749-6632.1981.tb33771.x [Crossref] [ Google Scholar]
- Estournet B. Plasma exchange in the treatment of myasthenia gravis. Intensive Care Med 1979; 5(4):203. doi: 10.1007/bf01683938 [Crossref] [ Google Scholar]
- Newsom-Davis J, Vincent A. Combined plasma exchange and immunosuppression in myasthenia gravis. Lancet 1979; 2(8144):688. doi: 10.1016/s0140-6736(79)92081-6 [Crossref] [ Google Scholar]
- Behan PO, Shakir RA, Simpson JA, Burnett AK, Allan TL, Haase G. Plasma-exchange combined with immunosuppressive therapy in myasthenia gravis. Lancet 1979; 2(8140):438-40. doi: 10.1016/s0140-6736(79)91492-2 [Crossref] [ Google Scholar]
- Pinching AJ, Peters DK, Davis JN. Plasma exchange in myasthenia gravis. Lancet 1977; 1(8008):428-9. doi: 10.1016/s0140-6736(77)92639-3 [Crossref] [ Google Scholar]
- Finn R, Coates PM. Plasma exchange in myasthenia gravis. Lancet 1977; 1(8004):190-1. doi: 10.1016/s0140-6736(77)91782-2 [Crossref] [ Google Scholar]
- Hilkevich O, Drory VE, Chapman J, Korczyn AD. The use of intravenous immunoglobulin as maintenance therapy in myasthenia gravis. Clin Neuropharmacol 2001; 24(3):173-6. doi: 10.1097/00002826-200105000-00010 [Crossref] [ Google Scholar]
- Huang CS, Hsu HS, Kao KP, Huang MH, Huang BS. Intravenous immunoglobulin in the preparation of thymectomy for myasthenia gravis. Acta Neurol Scand 2003; 108(2):136-8. doi: 10.1034/j.1600-0404.2003.00131.x [Crossref] [ Google Scholar]
- Ortiz-Salas P, Velez-Van-Meerbeke A, Galvis-Gomez CA, Rodriguez QJ. Human immunoglobulin versus plasmapheresis in Guillain-Barre syndrome and myasthenia gravis: a meta-analysis. J Clin Neuromuscul Dis 2016; 18(1):1-11. doi: 10.1097/cnd.0000000000000119 [Crossref] [ Google Scholar]
- Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Myasthenia Gravis Clinical Study Group. Ann Neurol 1997; 41(6):789-96. doi: 10.1002/ana.410410615 [Crossref] [ Google Scholar]
- Qureshi AI, Choudhry MA, Akbar MS, Mohammad Y, Chua HC, Yahia AM. Plasma exchange versus intravenous immunoglobulin treatment in myasthenic crisis. Neurology 1999; 52(3):629-32. doi: 10.1212/wnl.52.3.629 [Crossref] [ Google Scholar]
- Barth D, Nabavi Nouri M, Ng E, Nwe P, Bril V. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 2011; 76(23):2017-23. doi: 10.1212/WNL.0b013e31821e5505 [Crossref] [ Google Scholar]
- Sonett JR, Jaretzki A, 3rd 3rd. Thymectomy for nonthymomatous myasthenia gravis: a critical analysis. Ann N Y Acad Sci 2008; 1132:315-28. doi: 10.1196/annals.1405.004 [Crossref] [ Google Scholar]
- Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(1):7-15. doi: 10.1212/wnl.55.1.7 [Crossref] [ Google Scholar]
- Cea G, Benatar M, Verdugo RJ, Salinas RA. Thymectomy for non-thymomatous myasthenia gravis. Cochrane Database Syst Rev 2013(10):CD008111. doi: 10.1002/14651858.CD008111.pub2 [Crossref]
- Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 2016; 375(6):511-22. doi: 10.1056/NEJMoa1602489 [Crossref] [ Google Scholar]
- Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol 2019; 18(3):259-68. doi: 10.1016/s1474-4422(18)30392-2 [Crossref] [ Google Scholar]
- Benveniste MF, Rosado-de-Christenson ML, Sabloff BS, Moran CA, Swisher SG, Marom EM. Role of imaging in the diagnosis, staging, and treatment of thymoma. Radiographics 2011; 31(7):1847-61; discussion 61. doi: 10.1148/rg.317115505 [Crossref] [ Google Scholar]
- Jaretzki A, Steinglass KM, Sonett JR. Thymectomy in the management of myasthenia gravis. Semin Neurol 2004; 24(1):49-62. doi: 10.1055/s-2004-829596 [Crossref] [ Google Scholar]
- Skeie GO, Apostolski S, Evoli A, Gilhus NE, Illa I, Harms L. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol 2010; 17(7):893-902. doi: 10.1111/j.1468-1331.2010.03019.x [Crossref] [ Google Scholar]
- Kumar V, Kaminski HJ. Treatment of myasthenia gravis. Curr Neurol Neurosci Rep 2011; 11(1):89-96. doi: 10.1007/s11910-010-0151-1 [Crossref] [ Google Scholar]
- Seyfari B, Fatehi F, Shojaiefard A, Jafari M, Ghorbani-Abdehgah A, Nasiri S. Clinical outcome of thymectomy in myasthenia gravis patients: A report from Iran. Iran J Neurol 2018; 17(1):1-5. [ Google Scholar]
- Yablonsky P, Pischik V, Tovbina MG, Atiukov M. The results of video-assisted thoracoscopic thymectomies in Saint Petersburg, Russia: 20-year of experience. J Vis Surg 2017; 3:113. doi: 10.21037/jovs.2017.06.13 [Crossref] [ Google Scholar]
- Meyer DM, Herbert MA, Sobhani NC, Tavakolian P, Duncan A, Bruns M. Comparative clinical outcomes of thymectomy for myasthenia gravis performed by extended transsternal and minimally invasive approaches. Ann Thorac Surg 2009; 87(2):385-90. doi: 10.1016/j.athoracsur.2008.11.040 [Crossref] [ Google Scholar]
- Kim JY, Park KD, Richman DP. Treatment of myasthenia gravis based on its immunopathogenesis. J Clin Neurol 2011; 7(4):173-83. doi: 10.3988/jcn.2011.7.4.173 [Crossref] [ Google Scholar]
- Arsura E, Brunner NG, Namba T, Grob D. High-dose intravenous methylprednisolone in myasthenia gravis. Arch Neurol 1985; 42(12):1149-53. doi: 10.1001/archneur.1985.04060110031011 [Crossref] [ Google Scholar]
- Palace J, Newsom-Davis J, Lecky B. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Myasthenia Gravis Study Group. Neurology 1998; 50(6):1778-83. doi: 10.1212/wnl.50.6.1778 [Crossref] [ Google Scholar]
- Muscle Study Group. A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology 2008; 71(6):394-9. doi: 10.1212/01.wnl.0000312373.67493.7f [Crossref] [ Google Scholar]
- Sanders DB, Hart IK, Mantegazza R, Shukla SS, Siddiqi ZA, De Baets MH. An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology 2008; 71(6):400-6. doi: 10.1212/01.wnl.0000312374.95186.cc [Crossref] [ Google Scholar]
- Pasnoor M, He J, Herbelin L, Burns TM, Nations S, Bril V. A randomized controlled trial of methotrexate for patients with generalized myasthenia gravis. Neurology 2016; 87(1):57-64. doi: 10.1212/wnl.0000000000002795 [Crossref] [ Google Scholar]
- Zhou L, Liu W, Li W, Li H, Zhang X, Shang H. Tacrolimus in the treatment of myasthenia gravis in patients with an inadequate response to glucocorticoid therapy: randomized, double-blind, placebo-controlled study conducted in China. Ther Adv Neurol Disord 2017; 10(9):315-25. doi: 10.1177/1756285617721092 [Crossref] [ Google Scholar]
- De Feo LG, Schottlender J, Martelli NA, Molfino NA. Use of intravenous pulsed cyclophosphamide in severe, generalized myasthenia gravis. Muscle Nerve 2002; 26(1):31-6. doi: 10.1002/mus.10133 [Crossref] [ Google Scholar]
- Tindall RS, Phillips JT, Rollins JA, Wells L, Hall K. A clinical therapeutic trial of cyclosporine in myasthenia gravis. Ann N Y Acad Sci 1993; 681:539-51. doi: 10.1111/j.1749-6632.1993.tb22937.x [Crossref] [ Google Scholar]
- Tindall RS, Rollins JA, Phillips JT, Greenlee RG, Wells L, Belendiuk G. Preliminary results of a double-blind, randomized, placebo-controlled trial of cyclosporine in myasthenia gravis. N Engl J Med 1987; 316(12):719-24. doi: 10.1056/nejm198703193161205 [Crossref] [ Google Scholar]
- Tandan R, Hehir MK 2nd, Waheed W, Howard DB. Rituximab treatment of myasthenia gravis: A systematic review. Muscle Nerve 2017; 56(2):185-196. doi: 10.1002/mus.25597 [Crossref] [ Google Scholar]
- Dos Santos A, Noury JB, Genestet S, Nadaj-Pakleza A, Cassereau J, Baron C. Efficacy and safety of rituximab in myasthenia gravis: a French multicentre real-life study. Eur J Neurol 2020; 27(11):2277-85. doi: 10.1111/ene.14391 [Crossref] [ Google Scholar]
- Turner B, Cree BAC, Kappos L, Montalban X, Papeix C, Wolinsky JS. Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis. J Neurol 2019; 266(5):1182-93. doi: 10.1007/s00415-019-09248-6 [Crossref] [ Google Scholar]
- Howard JF Jr, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol 2017; 16(12):976-86. doi: 10.1016/s1474-4422(17)30369-1 [Crossref] [ Google Scholar]
- McKeage K. Ravulizumab: first global approval. Drugs 2019; 79(3):347-52. doi: 10.1007/s40265-019-01068-2 [Crossref] [ Google Scholar]
- Howard JF Jr, Nowak RJ, Wolfe GI, Freimer ML, Vu TH, Hinton JL. Clinical effects of the self-administered subcutaneous complement inhibitor zilucoplan in patients with moderate to severe generalized myasthenia gravis: results of a phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. JAMA Neurol 2020; 77(5):582-92. doi: 10.1001/jamaneurol.2019.5125 [Crossref] [ Google Scholar]
- Howard JF Jr, Bril V, Burns TM, Mantegazza R, Bilinska M, Szczudlik A. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology 2019; 92(23):e2661-e73. doi: 10.1212/wnl.0000000000007600 [Crossref] [ Google Scholar]
- Cartwright SL, Cartwright MS. Health maintenance for adults with neuromuscular diseases on immunosuppression. Muscle Nerve 2019; 59(4):397-403. doi: 10.1002/mus.26382 [Crossref] [ Google Scholar]
- Kerty E, Elsais A, Argov Z, Evoli A, Gilhus NE. EFNS/ENS Guidelines for the treatment of ocular myasthenia. Eur J Neurol 2014; 21(5):687-93. doi: 10.1111/ene.12359 [Crossref] [ Google Scholar]
- Wong SH, Huda S, Vincent A, Plant GT. Ocular myasthenia gravis: controversies and updates. Curr Neurol Neurosci Rep 2014; 14(1):421. doi: 10.1007/s11910-013-0421-9 [Crossref] [ Google Scholar]
- Benatar M, McDermott MP, Sanders DB, Wolfe GI, Barohn RJ, Nowak RJ. Efficacy of prednisone for the treatment of ocular myasthenia (EPITOME): a randomized, controlled trial. Muscle Nerve 2016; 53(3):363-9. doi: 10.1002/mus.24769 [Crossref] [ Google Scholar]
- Evoli A, Padua L. Diagnosis and therapy of myasthenia gravis with antibodies to muscle-specific kinase. Autoimmun Rev 2013; 12(9):931-5. doi: 10.1016/j.autrev.2013.03.004 [Crossref] [ Google Scholar]
- Evoli A, Tonali PA, Padua L, Monaco ML, Scuderi F, Batocchi AP. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003; 126(Pt 10):2304-11. doi: 10.1093/brain/awg223 [Crossref] [ Google Scholar]
- Lauriola L, Ranelletti F, Maggiano N, Guerriero M, Punzi C, Marsili F. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology 2005; 64(3):536-8. doi: 10.1212/01.wnl.0000150587.71497.b6 [Crossref] [ Google Scholar]
- Leite MI, Ströbel P, Jones M, Micklem K, Moritz R, Gold R. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol 2005; 57(3):444-8. doi: 10.1002/ana.20386 [Crossref] [ Google Scholar]
- Oh SJ. Muscle-specific receptor tyrosine kinase antibody positive myasthenia gravis current status. J Clin Neurol 2009; 5(2):53-64. doi: 10.3988/jcn.2009.5.2.53 [Crossref] [ Google Scholar]
- Evoli A, Bianchi MR, Riso R, Minicuci GM, Batocchi AP, Servidei S. Response to therapy in myasthenia gravis with anti-MuSK antibodies. Ann N Y Acad Sci 2008; 1132:76-83. doi: 10.1196/annals.1405.012 [Crossref] [ Google Scholar]
- Hehir MK, Hobson-Webb LD, Benatar M, Barnett C, Silvestri NJ, Howard JF Jr. Rituximab as treatment for anti-MuSK myasthenia gravis: multicenter blinded prospective review. Neurology 2017; 89(10):1069-77. doi: 10.1212/wnl.0000000000004341 [Crossref] [ Google Scholar]
- Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol 2011; 69(2):418-22. doi: 10.1002/ana.22312 [Crossref] [ Google Scholar]
- Kaminski HJ. Myasthenia Gravis and Related Disorders. Totowa, NJ: Humana Press; 2009.
- Romi F. Thymoma in myasthenia gravis: from diagnosis to treatment. Autoimmune Dis 2011; 2011:474512. doi: 10.4061/2011/474512 [Crossref] [ Google Scholar]
- Romi F, Skeie GO, Aarli JA, Gilhus NE. Muscle autoantibodies in subgroups of myasthenia gravis patients. J Neurol 2000; 247(5):369-75. doi: 10.1007/s004150050604 [Crossref] [ Google Scholar]
- Cheney RT. The biologic spectrum of thymic epithelial neoplasms: current status and future prospects. J Natl Compr Canc Netw 2010; 8(11):1322-8. doi: 10.6004/jnccn.2010.0097 [Crossref] [ Google Scholar]
- Kumar R. Myasthenia gravis and thymic neoplasms: a brief review. World J Clin Cases 2015; 3(12):980-3. doi: 10.12998/wjcc.v3.i12.980 [Crossref] [ Google Scholar]
- Romi F, Aarli JA, Gilhus NE. Myasthenia gravis patients with ryanodine receptor antibodies have distinctive clinical features. Eur J Neurol 2007; 14(6):617-20. doi: 10.1111/j.1468-1331.2007.01785.x [Crossref] [ Google Scholar]
- Zahid I, Sharif S, Routledge T, Scarci M. Video-assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis?. Interact Cardiovasc Thorac Surg 2011; 12(1):40-6. doi: 10.1510/icvts.2010.251041 [Crossref] [ Google Scholar]
- Erşen E, Kılıç B, Kara HV, İşcan M, Sarbay İ, Demirkaya A. Comparative study of video-assisted thoracoscopic surgery versus open thymectomy for thymoma and myasthenia gravis. Wideochir Inne Tech Maloinwazyjne 2018; 13(3):376-82. doi: 10.5114/wiitm.2018.75835 [Crossref] [ Google Scholar]
- Fujii Y. Published guidelines for management of thymoma. Thorac Surg Clin 2011; 21(1):125-9. doi: 10.1016/j.thorsurg.2010.08.002 [Crossref] [ Google Scholar]
- Falkson CB, Bezjak A, Darling G, Gregg R, Malthaner R, Maziak DE. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol 2009; 4(7):911-9. doi: 10.1097/jto.0b013e3181a4b8e0 [Crossref] [ Google Scholar]
- Evoli A, Batocchi AP, Minisci C, Di Schino C, Tonali P. Clinical characteristics and prognosis of myasthenia gravis in older people. J Am Geriatr Soc 2000; 48(11):1442-8. doi: 10.1111/j.1532-5415.2000.tb02635.x [Crossref] [ Google Scholar]
- Shimizu Y, Kitagawa K. Management of myasthenia gravis in pregnancy. Clin Exp Neuroimmunol 2016; 7(2):199-204. doi: 10.1111/cen3.12305 [Crossref] [ Google Scholar]
- Freyer AM. Drugs in pregnancy and lactation 8th edition: a reference guide to fetal and neonatal risk. Obstet Med 2009; 2(2):89. doi: 10.1258/om.2009.090002 [Crossref] [ Google Scholar]
- Stafford IP, Dildy GA. Myasthenia gravis and pregnancy. Clin Obstet Gynecol 2005; 48(1):48-56. doi: 10.1097/01.grf.0000153206.85996.07 [Crossref] [ Google Scholar]
- Norwood F, Dhanjal M, Hill M, James N, Jungbluth H, Kyle P. Myasthenia in pregnancy: best practice guidelines from a U.K. multispecialty working group. J Neurol Neurosurg Psychiatry 2014; 85(5):538-43. doi: 10.1136/jnnp-2013-305572 [Crossref] [ Google Scholar]
- Ostensen M, Brucato A, Carp H, Chambers C, Dolhain RJ, Doria A. Pregnancy and reproduction in autoimmune rheumatic diseases. Rheumatology (Oxford) 2011; 50(4):657-64. doi: 10.1093/rheumatology/keq350 [Crossref] [ Google Scholar]
- Sau A, Clarke S, Bass J, Kaiser A, Marinaki A, Nelson-Piercy C. Azathioprine and breastfeeding: is it safe?. Bjog 2007; 114(4):498-501. doi: 10.1111/j.1471-0528.2006.01232.x [Crossref] [ Google Scholar]
- Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006; 82(12):1698-702. doi: 10.1097/01.tp.0000252683.74584.29 [Crossref] [ Google Scholar]
- Bar Oz B, Hackman R, Einarson T, Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation 2001; 71(8):1051-5. doi: 10.1097/00007890-200104270-00006 [Crossref] [ Google Scholar]
- Kainz A, Harabacz I, Cowlrick IS, Gadgil SD, Hagiwara D. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation 2000; 70(12):1718-21. doi: 10.1097/00007890-200012270-00010 [Crossref] [ Google Scholar]
- Ferrero S, Pretta S, Nicoletti A, Petrera P, Ragni N. Myasthenia gravis: management issues during pregnancy. Eur J Obstet Gynecol Reprod Biol 2005; 121(2):129-38. doi: 10.1016/j.ejogrb.2005.01.002 [Crossref] [ Google Scholar]
- Varner M. Myasthenia gravis and pregnancy. Clin Obstet Gynecol 2013; 56(2):372-81. doi: 10.1097/GRF.0b013e31828e92c0 [Crossref] [ Google Scholar]
- Jacob S, Muppidi S, Guidon A, Guptill J, Hehir M, Howard JF Jr. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci 2020; 412:116803. doi: 10.1016/j.jns.2020.116803 [Crossref] [ Google Scholar]
- Hübers A, Lascano AM, Lalive PH. Management of patients with generalised myasthenia gravis and COVID-19: four case reports. J Neurol Neurosurg Psychiatry 2020; 91(10):1124-5. doi: 10.1136/jnnp-2020-323565 [Crossref] [ Google Scholar]
- Aksoy E, Oztutgan T. COVID-19 presentation in association with myasthenia gravis: a case report and review of the literature. Case Rep Infect Dis 2020; 2020:8845844. doi: 10.1155/2020/8845844 [Crossref] [ Google Scholar]
- Costamagna G, Abati E, Bresolin N, Comi GP, Corti S. Management of patients with neuromuscular disorders at the time of the SARS-CoV-2 pandemic. J Neurol 2021; 268(5):1580-91. doi: 10.1007/s00415-020-10149-2 [Crossref] [ Google Scholar]
- Hoff JM, Daltveit AK, Gilhus NE. Myasthenia gravis in pregnancy and birth: identifying risk factors, optimising care. Eur J Neurol 2007; 14(1):38-43. doi: 10.1111/j.1468-1331.2006.01538.x [Crossref] [ Google Scholar]
- Béhin A, Mayer M, Kassis-Makhoul B, Jugie M, Espil-Taris C, Ferrer X. Severe neonatal myasthenia due to maternal anti-MuSK antibodies. Neuromuscul Disord 2008; 18(6):443-6. doi: 10.1016/j.nmd.2008.03.006 [Crossref] [ Google Scholar]
- Kanzaki A, Motomura M. [A pregnant patient with anti-MuSK antibody positive myasthenia gravis and her infant with transient neonatal myasthenia gravis]. Rinsho Shinkeigaku 2011; 51(3):188-91. doi: 10.5692/clinicalneurol.51.188.[Japanese] [Crossref] [ Google Scholar]
- Strijbos E, Huijbers MG, van Es IE, Alleman I, van Ostaijen-Ten Dam MM, Bakker J. A prospective, placebo controlled study on the humoral immune response to and safety of tetanus revaccination in myasthenia gravis. Vaccine 2017; 35(46):6290-6. doi: 10.1016/j.vaccine.2017.09.078 [Crossref] [ Google Scholar]
- Csuka D, Czirják L, Hóbor R, Illes Z, Bánáti M, Rajczy K. Effective humoral immunity against diphtheria and tetanus in patients with systemic lupus erythematosus or myasthenia gravis. Mol Immunol 2013; 54(3-4):453-6. doi: 10.1016/j.molimm.2013.01.012 [Crossref] [ Google Scholar]
- Tackenberg B, Schneider M, Blaes F, Eienbröker C, Schade-Brittinger C, Wellek A. Acetylcholine receptor antibody titers and clinical course after influenza vaccination in patients with myasthenia gravis: a double-blind randomized controlled trial (ProPATIent-Trial). EBioMedicine 2018; 28:143-50. doi: 10.1016/j.ebiom.2018.01.007 [Crossref] [ Google Scholar]
- Seok HY, Shin HY, Kim JK, Kim BJ, Oh J, Suh BC. The impacts of influenza infection and vaccination on exacerbation of myasthenia gravis. J Clin Neurol 2017; 13(4):325-30. doi: 10.3988/jcn.2017.13.4.325 [Crossref] [ Google Scholar]
- Gilhus NE, Romi F, Hong Y, Skeie GO. Myasthenia gravis and infectious disease. J Neurol 2018; 265(6):1251-8. doi: 10.1007/s00415-018-8751-9 [Crossref] [ Google Scholar]
- Mehrizi M, Fontem RF, Gearhart TR, Pascuzzi RM. Medications and Myasthenia Gravis (A Reference for Health Care Professionals). Myasthenia Gravis Foundation of America; 2012. p. 1-49.
- Rath J, Mauritz M, Zulehner G, Hilger E, Cetin H, Kasprian G. Iodinated contrast agents in patients with myasthenia gravis: a retrospective cohort study. J Neurol 2017; 264(6):1209-17. doi: 10.1007/s00415-017-8518-8 [Crossref] [ Google Scholar]
- El-Bawab H, Hajjar W, Rafay M, Bamousa A, Khalil A, Al-Kattan K. Plasmapheresis before thymectomy in myasthenia gravis: routine versus selective protocols. Eur J Cardiothorac Surg 2009; 35(3):392-7. doi: 10.1016/j.ejcts.2008.11.006 [Crossref] [ Google Scholar]
- Saeteng S, Tantraworasin A, Siwachat S, Lertprasertsuke N, Euathrongchit J, Wannasopha Y. Preoperative plasmapheresis for elective thymectomy in myasthenia patient: is it necessary?. ISRN Neurol 2013; 2013:238783. doi: 10.1155/2013/238783 [Crossref] [ Google Scholar]
- Blichfeldt-Lauridsen L, Hansen BD. Anesthesia and myasthenia gravis. Acta Anaesthesiol Scand 2012; 56(1):17-22. doi: 10.1111/j.1399-6576.2011.02558.x [Crossref] [ Google Scholar]
- Romero A, Joshi GP. Neuromuscular disease and anesthesia. Muscle Nerve 2013; 48(3):451-60. doi: 10.1002/mus.23817 [Crossref] [ Google Scholar]
- Crespo V, James ML. Neuromuscular disease in the neurointensive care unit. Anesthesiol Clin 2016; 34(3):601-19. doi: 10.1016/j.anclin.2016.04.010 [Crossref] [ Google Scholar]