Arch Iran Med. 25(2):78-84.
doi: 10.34172/aim.2022.13
Original Article
Effect of Post IORT Wound Fluid Secretion (PIWFS) on the Behavior of Breast Cancer Cells: Stimulator or Inhibitor; Report of an Experimental Study on Breast Cancer
Seyed Mohammadreza Javadi 1  , Mohammad Abdolahad 2, Solmaz Hashemi 3, Mohammadali Khayamian 2, Mohammad Salemizadeh Parizi 2, Shohreh Vanaei 2, Hamidreza Mirzaei 4, Shabnam Jeibouei 5, Ali Hojat 4, Hakimeh Zali 6, Seied Rabi Mahdavi 7, Mohammad Esmaeil Akbari 4, *
, Mohammad Abdolahad 2, Solmaz Hashemi 3, Mohammadali Khayamian 2, Mohammad Salemizadeh Parizi 2, Shohreh Vanaei 2, Hamidreza Mirzaei 4, Shabnam Jeibouei 5, Ali Hojat 4, Hakimeh Zali 6, Seied Rabi Mahdavi 7, Mohammad Esmaeil Akbari 4, * 
Author information:
1Hamadan University of Medical Sciences, General Surgery Department, Hamadan, Iran
2Nano Bio Electronic Devices Lab, Cancer Electronics Research Group, School of Electrical and Computer Engineering, University of Tehran, Tehran, Iran
3General Surgery Department, Tabriz University of Medical Sciences, Tabriz, Iran
4Cancer Research Center, Shahid Beheshti Medical University of Medical Sciences, Tehran, Iran
5Department of Biotechnology, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
6Department of Tissue Engineering and Applied Cell Sciences, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
7Medical Physics Department, Iran University of Medical Sciences, Tehran, Iran
*Corresponding Author: Mohammad Esmail Akbari, MD; Head of Cancer Research Center, Manager of Cancer & Breast surgery fellowship in Iran. Cancer Research Center. Shahrdari st., Tehran, I.R. Iran. Tel: +98(21)22748001-2, Fax: +98(21)22724090; Email:
crc@gmail.com;
profmeakbari@gmail.com
Abstract
Background:
Although investigating the probable side effects of post intraoperative radiotherapy wound fluid secretion (PIWFS) is crucial, especially in clinical cases, no report has been published on the effect of PIWFS on the remaining tumor cells (in the vital state) in cavity side margins or surrounding regions. These tumor cells might be directly/indirectly exposed to intraoperative radiation therapy (IORT). Here, for the first time, we investigated the effect of PIWFS on tumor cells of the same patient extracted from the excised tumor in the spheroid form.
Methods:
We generated 8 human-derived breast tumor spheroids from 4 patient specimens who received to IORT, dissociated and cultured them in microfluidic devices. The spheroids from each sample were treated with the patients’ PIWFS and DMEM medium separately. Two different parameters, called area and number of detached cells (NDCs), were determined and investigated to evaluate the spheroids’ vital and proliferative states.
Results:
The results showed severe transformation in tumor spheroids’ function into more invasive and proliferative functions after treatment with PIWFS.
Conclusion:
Although the radiation-induced bystander effect may have a role in this observation, further experiments must be done to better clarify the probable desired or non-desired effects of post-IORT secretion for both the remaining tumor cells and the surrounding immune cells.
Keywords: Breast cancer, Cell culture, IORT, Microfluidics, Wound fluid
Copyright and License Information
© 2022 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Javadi SM, Abdolahad M, Hashemi S, Khayamian M, Salemizadeh Parizi M, Vanaei S, et al. Effect of Post IORT wound fluid secretion (PIWFS) on the behavior of breast cancer cells: stimulator or inhibitor; report of an experimental study on breast cancer. Arch Iran Med. 2022;25(2):78-84. doi: 10.34172/aim.2022.13
Introduction
Today, intraoperative radiation therapy (IORT) has gained worldwide acceptance as part of the standard treatment for early-stage breast cancer in women undergoing breast-conserving surgery (BCS).1,2 IORT is defined as a single dose of irradiation delivered to the tumor bed at the surgery time and can be substituted for whole-breast irradiation. By applying a suitable applicator during BCS, a high single-dose energy of electron3 or X-ray4,5 is delivered to the tumor bed. In patients with unsuitable or cautionary criteria to receive the full dose, IORT is prescribed as an alternative to the external boost dose.6,7 Previously, targeted intraoperative radiotherapy (TARGIT)1 and electron beam intraoperative radiotherapy (ELIOT)3 trials have been carried out on a large group of patients, and some trials have indicated that IORT-treated patients had no worse overall survival (OS) and disease‐free survival (DFS) than those treated with external beam radiation therapy (EBRT).4-9
Even using boost doses of IORT in patients with breast cancer [both invasive ductal and lobular carcinoma (IDC and ILC)] or delayed targeted intraoperative radiotherapy (TARGIT-IORT) (i.e., the site of tumor surgery was reopened in a separate surgery and radiotherapy performed) may lead to acceptable results in terms of local recurrence or metastasis (compared to applying EBRT alone).8,9
Although IORT has been shown to yield acceptable results compared with EBRT,10 its significant effect in the tumor bed’s microenvironment raises many concerns about both desired and non-desired effects of localized radiation on cellular activities, either probably remaining tumor cells or surrounding immune cells.
One of the mediators or symptoms of such changes might be the wound fluid secreted in the tumor cavity post-IORT as it contains a wide variety of secreted proteins and enzymes that may favor or counter tumor cells҆ vitality. Therefore, extensive research has been conducted on the effect of post-IORT wound fluid secretion (PIWFS) on breast cancer cells and its comparison with the wound fluid of the IORT-free BCS cases.11
Inconclusive results have been published about the effects of PIWFS, ranging from disrupting proliferation and mutation of cancer cells to non-perturbing effects on both cancer and normal cells. Radiobiological and bystander effects have been reported as the main reasons underlying the cancer-disturbing effects of PIWFS.11-14
Several publications have revealed that IORT-treated (boost, radical, and X-ray) seroma significantly affects proliferation, cell cycle arrest, death, migration, and invasion of the cancer cells. Besides, another valuable study based on functional annotation and gene ontology indicated that significant enrichment in molecular pathways on breast-conserving treatment is somehow independent of single high-dose radiation, meaning that crucial molecular pathways in IORT are equally enriched by both boost and radical doses.15
Other studies have reported that the cancer destructive effects of PIWFS might be related to secretory products of the immune system recalled to the treated lesion to exert functional effects (such as the radiation-induced bystander effect) in host margin cells by indirect radiation.13
Although investigating the probable side effects of PIWFS is crucial, especially in clinical cases, no report has been published on the effect of PIWFS on tumor cells of the same patient that might have remained in cavity side margins or surrounding regions. These tumor cells (in the case of surrounding margin cells) might be directly/indirectly exposed to IORT.
For experimentally simulating such evidence, a fresh tumor species was removed from the patients and was located on the lateral side of IORT emission so that there was a tumor in the bystander condition. After termination of IORT, the sample was divided into two individual parts, and vital spheroids were made from them. Each sample was then individually introduced to PIWFS of the same patient and a standard culture media.
This study is of high importance as many reports have revealed that in some lumpectomy or partial mastectomy cases, at least satellite lesions of atypical ductal hyperplasia or ductal carcinoma in-situ might remain in surgical cavity side margins (although missed in frozen sections).12,16-19 These tumor cells might not even have a trace in the permanent pathology of tumor side margins, and if they become activated by PIWFS, the probability of tumor recurrence might be increased.
Materials and Methods
Study Design
This study was done based on sampling from patients with breast cancer who were candidates for BCS and had undergone IORT. The Iranian intraoperative radiotherapy (IRIORT) consensus (Supplementary file 1) was used to prescribe radical or boost dose of IORT. Patients who underwent systemic neoadjuvant therapy were not included in the study. A radical dose of 21 Gy and a boost dose of 12 Gy were selected.
After BCS and removing a fresh species of tumor from the patients, the tumor sample was transferred to the pathology department, and if the tumor sampling did not interfere with the process of preparing diagnostic samples, a sample (5 × 5 mm with a thickness of 1 mm) was also separated from the tissue. Then, the sample was divided into two parts. One part was immediately transferred to Falcon tubes containing the standard solution, and the second sample was located on the lateral side of IORT emission.
So, there was a tumor in bystander condition. After termination of IORT, the sample was divided into two individual parts, and vital spheroids (prepared as described below) were made from them. Then, each sample was individually introduced to PIWFS (as below) and a standard culture media (Dulbecco’s modified eagles҆ medium (DMEM) + fetal bovine serum (FBS) 10% solution), respectively. Results were evaluated by time-lapse microscopic imaging from proliferation and migration of both spheroids by some newly defined figures of merits, such as the number of the cells detached from the spheroids or progression size of the spheroids.
Sample preparation and wound fluid (WF) collection were performed for non-IORT samples (surgery without radiotherapy) according to the defined method except for IORT.
Two different parameters, called area and number of detached cells (NDCs), were determined and investigated to evaluate the spheroids’ vital and proliferative states.
Spheroid Preparation
Briefly, according to the standard protocol for spheroid preparation, the sample was cut into sections with 40–100 μm dimensions using a mechanical method and a sterile scalpel and was then filtered using collagenase type 1. The sample was then transferred to AIM BIOTECHs҆ hydrogel medium (DAX-1, AIM BIOTECH, https://www.aimbiotech.com/) and placed in a special chamber incubator at a temperature of 37°C with suitable humidity and oxygen volume. Two spheroids were obtained from each sample (A as case and B as control). Furthermore, the DMEM + FBS10% solution was only added within the first 24 hours.
Wound Fluid Collection
After IORT and breast reconstruction, a closed tubular vacuum drain was placed in the tumor cavity and locked so that there were no secretion releases from the body during the first 24 hours. After 24 hours, the drain was unlocked, and wound secretions were collected in a sterile Falcon tube and were transferred to the laboratory by maintaining the cold chain and added to medium A after centrifugation and filtration. Medium B was still incubated by adding the DMEM + FBS10% solution.
Results
The four cases included in this investigation are introduced in Table 1 and comprise women with invasive ductal carcinoma (IDC) and double-positive phenotype (ER+, PR+, HER2:-).
Table 1.
Demographic and pathologic characteristics of the samples and spheroid indices
|
Patient ID
|
Age
|
Pathology
|
IHC
|
IORT
|
Spheroid Area
DMEM
|
Spheroid Area
WFS
|
No. of Detached Cells
DMEM
|
No. of Detached Cells
WFS
|
| #1 |
55 |
IDC |
ER: +
PR: +
HER2: -Ki67: 10-15% |
– |
0.3×102 µm2 |
0.4×102 µm2 |
0 |
0 |
| #2 |
55 |
IDC |
ER: +
PR: +
HER2: -Ki67: 5-10% |
12Gy |
0.12×102 µm2 |
1×102 µm2 |
0 |
5 |
| #3 |
49 |
IDC |
ER: +
PR: +
HER2:-Ki67:15-20% |
12Gy |
0.2×103 µm2 |
1.2×103 µm2 |
8 |
<40 |
| #4 |
46 |
IDC |
ER: +
PR: +
HER2: -Ki67: 15-20% |
12Gy |
0×102 µm2 |
1.5×102 µm2 |
0 |
4 |
IDC, Invasive ductal carcinoma; ER, Estrogen receptor; PR, Progesterone receptor; IORT, intraoperative radiation therapy; DMEM, Dulbecco’s modified eagles҆ medium; WFS, Wound Fluid Secretion.
Case ID #1 had IDC whose tumor spheroids were divided into two individual parts and were treated by the DMEM culture media and wound fluid seroma (obtained from the tumor cavity) of the patient, respectively (Figure 1). BCS had been carried out for the patient without IORT.
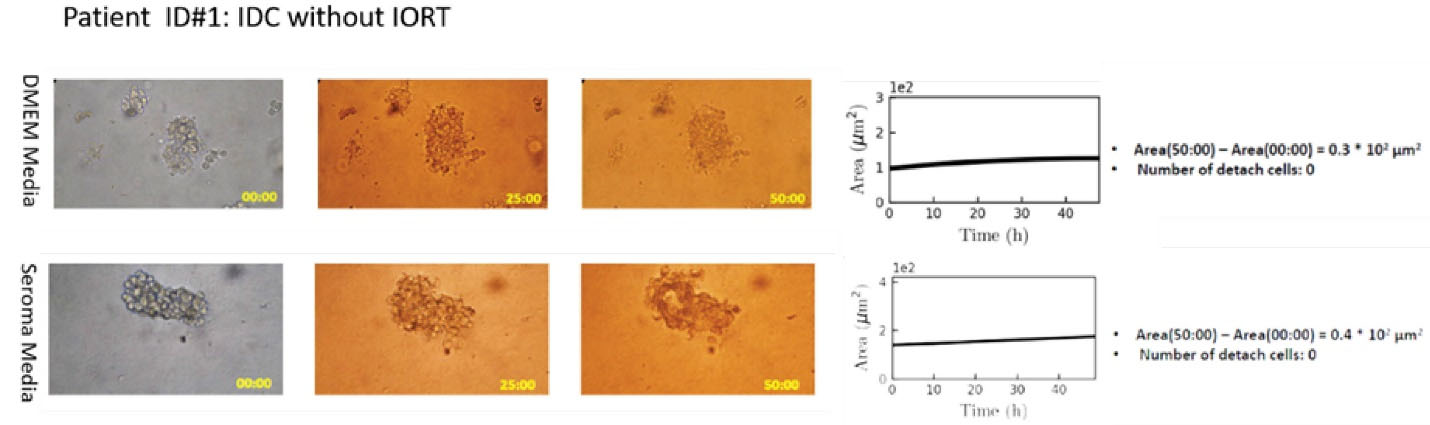
Figure 1.
Right panel: Microscopic image of tumor spheroid treated by the DMEM (top) and PIWFS (bottom) media over time. Left panel: Quantitative measurement of the size of spheroids over time. No drastic changes could be observed in spheroid progression between two media.
.
Right panel: Microscopic image of tumor spheroid treated by the DMEM (top) and PIWFS (bottom) media over time. Left panel: Quantitative measurement of the size of spheroids over time. No drastic changes could be observed in spheroid progression between two media.
There was no significant difference between the two spheroids treated by the DMEM or non-IORT WF media regarding the spheroid growth and the number of the cells detached from the spheroid (movie Supplement 1).
Case ID #2 was a patient with IDC who underwent IORT (dose: 12 Gy) during BCS. Individual spheroids of the patient’s tumor were separately treated with the DMEM and PIWFS media. Results presented in (Figure 2 and movie Supplement 2) show a further increment in SA and NDC for the spheroid treated by the PIWFS medium.
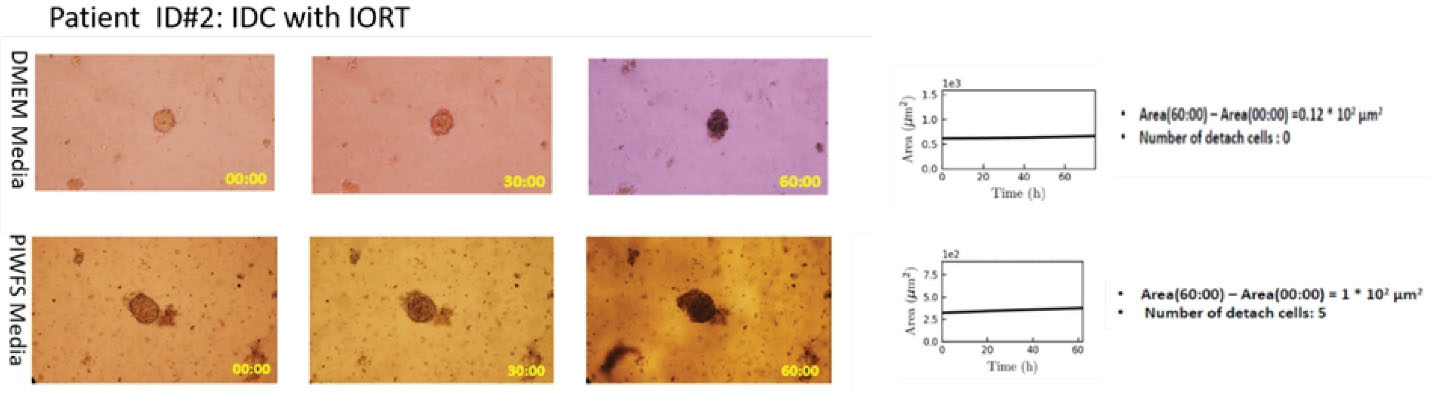
Figure 2.
Right panel: Microscopic image of tumor spheroid treated by DMEM (top) and PIWFS (bottom) media over time. Left panel: Quantitative measurement of the size of spheroids over time. PIWFS culture medium significantly stimulated progression and migration of tumor spheroid relative to the DMEM culture medium.
.
Right panel: Microscopic image of tumor spheroid treated by DMEM (top) and PIWFS (bottom) media over time. Left panel: Quantitative measurement of the size of spheroids over time. PIWFS culture medium significantly stimulated progression and migration of tumor spheroid relative to the DMEM culture medium.
Case ID #3 was a case similar to ID #2. Again, the patient’s therapeutic regimen was BCS along with IORT (12 Gy). Analyses of spheroids revealed further proliferation in the patient’s PIWFS medium relative to the DMEM medium (Figure 3). Such hyperactivation induced in PIWFS-treated spheroid was more significant than the similar spheroid from Case ID #2.
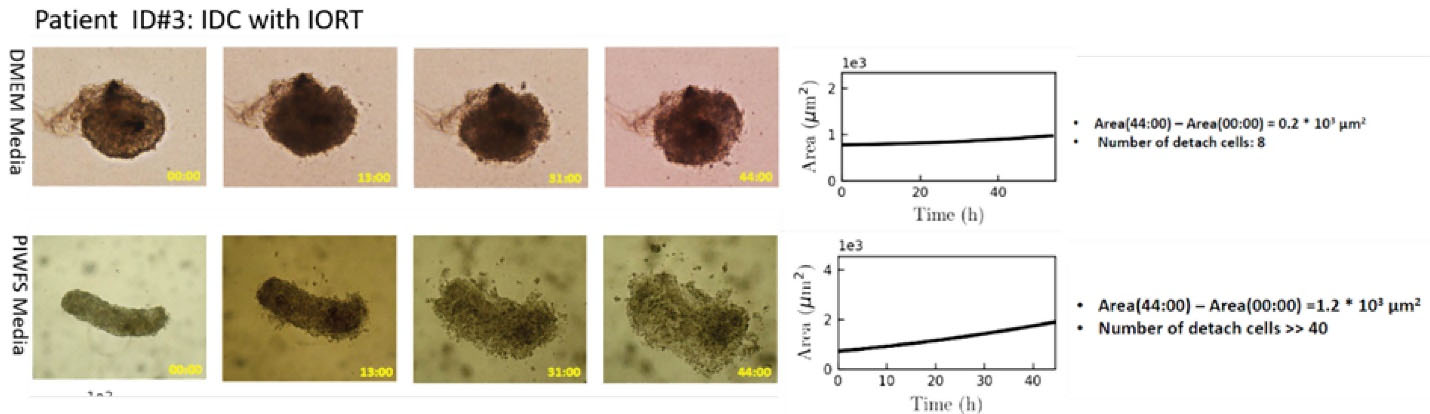
Figure 3.
Right panel: Microscopic image of tumor spheroid treated by DMEM (top) and PIWFS (bottom) media over time. Left panel: Quantitative measurement of the size of spheroids over time. PIWFS culture medium significantly stimulated progression and migration of tumor spheroid relative to the DMEM culture medium, which was much more significant than the progression observed for the spheroid of Case ID#2.
.
Right panel: Microscopic image of tumor spheroid treated by DMEM (top) and PIWFS (bottom) media over time. Left panel: Quantitative measurement of the size of spheroids over time. PIWFS culture medium significantly stimulated progression and migration of tumor spheroid relative to the DMEM culture medium, which was much more significant than the progression observed for the spheroid of Case ID#2.
Case ID #4 was a patient with IDC with similar phenotypes to Cases ID #2 and 3, but her spheroid showed no expansion or progressive functions in the DMEM culture medium (Figure 4), while the patient’s other spheroid treated with the patient’s PIWFS medium showed expanded size and hyperactivated detached and migrated cells from the spheroid (Figure 4). Such a distinct difference between the spheroids’ behavior in the DMEM and PIWFS media was not observed in the other patients. The results also revealed a significant increase in both the growth rate and the number of the isolated cells in the PIWFS medium.
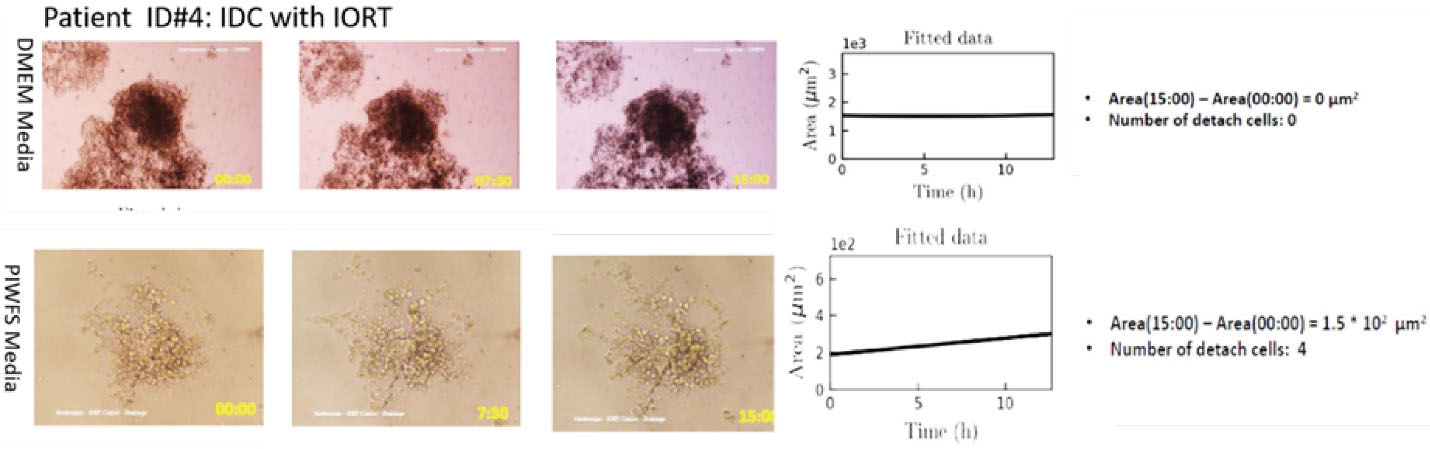
Figure 4.
Right panel: Microscopic image of tumor spheroid treated by DMEM (top) and PIWFS (bottom) media over time. Left panel: Quantitative measurement of the size of spheroids over time. PIWFS culture medium significantly stimulated progression and migration of tumor spheroid relative to DMEM culture medium, which was similar to the progression rate observed in the spheroid of Case ID#3.
.
Right panel: Microscopic image of tumor spheroid treated by DMEM (top) and PIWFS (bottom) media over time. Left panel: Quantitative measurement of the size of spheroids over time. PIWFS culture medium significantly stimulated progression and migration of tumor spheroid relative to DMEM culture medium, which was similar to the progression rate observed in the spheroid of Case ID#3.
In these four cases, the total mean spheroid growth was equal to 4.83 × 102µm2 after exposure to PIWFS medium, and 0.6 × 102 µm2 after exposure to the DMEM + FBS10% solution. The mean number of the isolated cells was also more than 50 cells in the sample exposed to the PIWFS medium while it was equal to 2 cells in the samples treated by the DMEM + FBS10% medium.
Discussion
IORT directly affects the destruction of residual cancer cells in the tumor bed and has many indirect effects, mostly reported to be in favor of therapy. An essential effect of radiotherapy is the modulation of the immune system by disrupting the tumor’s defense mechanism against the immune system through releasing tumor-associated antigens, activating natural killer cells (such as CD8+ cells), and secreting immune mediators, such as interferon-gamma.19-21 Besides, radiotherapy can lead to the secretion of a wide range of cytokines and other immune mediators by RT-targeted tumor cells and surrounding cells, such as tumor stromal endothelial cells and tumor-infiltrating lymphocytes.13
In addition to the “well-known” direct effect of IORT on tumor cells, indirect effects of radiation have also been found exerted on neighboring cells and leading to microenvironmental changes.13 There are numerous factors involved in this phenomenon, known as the bystander effect, including cell-to-cell gap junctions, reactive oxygen species, and reactive nitrogen species (e.g., nitric oxide synthase), and increment in the level of many cytokines and chemokines. Although some of them are in favor of destructing cancer cells,19,20 others may help in the activation and proliferation of the non-treated remaining cancer cells in the tumor bed (e.g., transforming growth factor-beta, tumor necrosis factor-alpha, and interleukin 6).11
Although some reports have revealed that microenvironmental changes have an inhibitory effect on tumor growth,13 some researchers believe that the bystander effect mediators may induce tumor growth.22
Since microscopic foci of cancer cells, stem cells, and a range of pre-cancerous cells from atypical to ductal intraepithelial neoplasia grade 3 are likely to persist around the cavity side margin or surrounding tissues (especially about one cm from the primary tumor), this seroma may be essential to be investigated in interaction with suspicious cells. It is important to investigate the interaction between PIWFS and vital tumor cells independently. If some pre-cancerous or cancerous cells have remained alive after IORT in the tumor bed, how would PIWFS influence their vitality without considering the interactive parameters between immune cells and remaining neoplastic cells?
The main difference between this study and the other previous studies is that the patient-derived tumor cells were incubated with wound fluid obtained from the same patient’s tumor bed.
Our findings showed that the patients’ tumor cell spheroid was reconstructed, and its invasive functions were improved in the presence of PIWFS as they underwent growth spurts, migrated, and formed new colonies (Figures 2–4). Growth and proliferation of tumor spheroids treated with the DMEM medium were lower than those treated with the PIWFS medium.
These findings are not consistent with those of other studies, showing the need for deeper investigation on the hidden effects of PIWFS, both on tumor recurrence or its better remission in response to IORT. Therefore, the following questions are set forth: Does the radiation-induced bystander effect always manifest itself in the form of tumor inhibition? What are the effects of cytokines and chemokines released during inflammation on neoplastic and immune cells? Will they have a cancer-suppressive or cancer-supportive role? The main experiment that will shed new light in this field is considering the patients’ surrounding immune cells’ response near their tumor cells in interaction with PIWFS. If the immune cells treated by PIWFS behave more aggressively in attacking the PIWFS- treated neoplastic cells, we can still hope for the therapeutic effects of PIWFS. Hence, further experiments must be done to elaborate the effect of cyto/chemokines in the presence of inflammatory environmental cells in the tumor bed in interaction with residual neoplastic cells, which is in progress by our research team.
In conclusion, our results showed that in the absence of immune cells, vital neoplastic cells showed further progressed proliferative functions in the PIWFS medium compared to a standard culture medium. As this research was carried out using the tumor cells of the patients in interaction with PIWFS of the same patient and similar results were observed in all three IDC cases (who had been treated by BCS and IORT), it is necessary to focus on the probable non-desired effects of PIWFS in clinical samples.
Although these results warn about the activation of remaining cancer cells in cavity side margins, the co-existence of the activated immune cells in the cavity near the stimulated remaining tumor cells should also be investigated. The immune cells triggered by cytokines in the surgical region might invade the remaining cancer cells, and PIWFS may destroy the remaining cancer cells. This hypothesis will also be investigated in our future research. Finally, we need to answer the important question whether the residual PIWFS in a tumor cavity is beneficial or harmful.
Supplementary Materials
Supplementary file 1 contains Figure S1.
(pdf)
Authors’ Contribution
MEA: Conceptualization, data curation, investigation, methodology, project administration, supervision, validation, visualization, writing – review & editing. SMJ: Conceptualization, data curation, investigation, methodology, validation, visualization, roles/writing – original draft. MA: Data curation, formal analysis, methodology, software, writing – review & editing. SH: Data curation, resources. MK: Formal analysis, investigation, resources; software, visualization. MSP: Formal analysis, investigation, resources, software, visualization. SV: Formal analysis, investigation, resources, software, visualization. HM: Investigation, methodology. SRM: Investigation, methodology. SJ: Investigation, methodology, validation, visualization. AH: Investigation, methodology, validation, visualization. HZ: Investigation, methodology, validation; visualization.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interest regarding the publication of this paper.
Ethical Statement
The ethical committee of Shahid Beheshti University of Medical Sciences approved this study
(IR.SBMU.CRC.REC.1398.025) and written informed consent was obtained from the patients or from parents or legal guardians for minors or incapacitated adults.
Data Availability
The data used to support this study’s findings, including supplementary movies, are available from the corresponding author upon request.
Funding
This research has been funded by “Cancer research center of Shahid Beheshti university of medical sciences”.
References
- Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014; 383(9917):603-13. doi: 10.1016/s0140-6736(13)61950-9 [Crossref] [ Google Scholar]
- Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009; 74(4):987-1001. doi: 10.1016/j.ijrobp.2009.02.031 [Crossref] [ Google Scholar]
- Orecchia R, Leonardi MC, Maisonneuve P, Morra A, Lazzari R, Cattani F. Intraoperative radiotherapy with electrons (ELIOT) for early breast cancer: the European Institute of Oncology experience. Transl Cancer Res 2014; 3(1):59-64. doi: 10.3978/j.issn.2218-676X.2014.02.04 [Crossref] [ Google Scholar]
- Vaidya J, Wenz F, Bulsara M, Joseph D, Tobias J, Keshtgar M. Abstract S4-2: Targeted intraoperative radiotherapy for early breast cancer: TARGIT-A trial- updated analysis of local recurrence and first analysis of survival. Cancer Res 2012; 72(24 Suppl):S4-2. doi: 10.1158/0008-5472.sabcs12-s4-2 [Crossref] [ Google Scholar]
- Corica T, Nowak AK, Saunders CM, Bulsara M, Taylor M, Vaidya JS. Cosmesis and breast-related quality of life outcomes after intraoperative radiation therapy for early breast cancer: a substudy of the TARGIT-A trial. Int J Radiat Oncol Biol Phys 2016; 96(1):55-64. doi: 10.1016/j.ijrobp.2016.04.024 [Crossref] [ Google Scholar]
- Sedlmayer F, Reitsamer R, Wenz F, Sperk E, Fussl C, Kaiser J. Intraoperative radiotherapy (IORT) as boost in breast cancer. Radiat Oncol 2017; 12(1):23. doi: 10.1186/s13014-016-0749-9 [Crossref] [ Google Scholar]
- Moini N, Nafissi N, Babaee E, Mirzaei HR, Akbari ME. Comparing the outcome of boost dose of intraoperative radiotherapy with electron (IOERT) and low-kV X-ray (IOXRT) and external beam radiotherapy (EBRT) in breast cancer after neoadjuvant chemotherapy. Int J Cancer Manag 2019; 12(8):e94547. doi: 10.5812/ijcm.94547 [Crossref] [ Google Scholar]
- Salati A, Akbari ME, Nafissi N, Noorian S, Mahdavi SR, Mirzaei HR. Comparison of outcome between invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC) patients treating with breast conserving surgery (BCS) and radical dose of intraoperative electron radiotherapy (IOERT). Int J Cancer Manag 2018; 11(11):e80985. doi: 10.5812/ijcm.80985 [Crossref] [ Google Scholar]
- Vaidya JS, Bulsara M, Saunders C, Flyger H, Tobias JS, Corica T. Effect of delayed targeted intraoperative radiotherapy vs whole-breast radiotherapy on local recurrence and survival: long-term results from the TARGIT-A randomized clinical trial in early breast cancer. JAMA Oncol 2020; 6(7):e200249. doi: 10.1001/jamaoncol.2020.0249 [Crossref] [ Google Scholar]
- Belletti B, Vaidya JS, D’Andrea S, Entschladen F, Roncadin M, Lovat F. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 2008; 14(5):1325-32. doi: 10.1158/1078-0432.ccr-07-4453 [Crossref] [ Google Scholar]
- Veldwijk MR, Neumaier C, Gerhardt A, Giordano FA, Sütterlin M, Herskind C. Comparison of the proliferative and clonogenic growth capacity of wound fluid from breast cancer patients treated with and without intraoperative radiotherapy. Transl Cancer Res 2015; 4(2):173-7. doi: 10.3978/j.issn.2218-676X.2015.04.01 [Crossref] [ Google Scholar]
- Sologuren I, Rodríguez-Gallego C, Lara PC. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Cancer Res 2014; 3(1):18-31. doi: 10.3978/j.issn.2218-676X.2014.02.05 [Crossref] [ Google Scholar]
- Kulcenty K, Piotrowski I, Zaleska K, Wichtowski M, Wróblewska J, Murawa D. Wound fluids collected postoperatively from patients with breast cancer induce epithelial to mesenchymal transition but intraoperative radiotherapy impairs this effect by activating the radiation-induced bystander effect. Sci Rep 2019; 9(1):7891. doi: 10.1038/s41598-019-44412-y [Crossref] [ Google Scholar]
- Piotrowski I, Kulcenty K, Murawa D, Suchorska W. Surgical wound fluids from patients treated with intraoperative radiotherapy induce radiobiological response in breast cancer cells. Med Oncol 2018; 36(2):14. doi: 10.1007/s12032-018-1243-z [Crossref] [ Google Scholar]
- Shahani M, Shakeri J, Akbari ME, Arefnezhad B, Tafti A, Zali H. Transcriptomic and proteomic approaches reveal biological basis of intraoperative radiotherapy-treated tumor bed modification in breast cancer patients: A pilot study. J Proteomics 2020; 212:103596. doi: 10.1016/j.jprot.2019.103596 [Crossref] [ Google Scholar]
- Baker JL, Hasteh F, Blair SL. Atypical ductal hyperplasia at the margin of lumpectomy performed for early stage breast cancer: is there enough evidence to formulate guidelines?. Int J Surg Oncol 2012; 2012:297832. doi: 10.1155/2012/297832 [Crossref] [ Google Scholar]
- Leong C, Boyages J, Jayasinghe UW, Bilous M, Ung O, Chua B. Effect of margins on ipsilateral breast tumor recurrence after breast conservation therapy for lymph node-negative breast carcinoma. Cancer 2004; 100(9):1823-32. doi: 10.1002/cncr.20153 [Crossref] [ Google Scholar]
- Pilewskie M, Morrow M. Margins in breast cancer: how much is enough?. Cancer 2018; 124(7):1335-41. doi: 10.1002/cncr.31221 [Crossref] [ Google Scholar]
- Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009; 15(17):5379-88. doi: 10.1158/1078-0432.ccr-09-0265 [Crossref] [ Google Scholar]
- Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol 2012; 2:191. doi: 10.3389/fonc.2012.00191 [Crossref] [ Google Scholar]
- Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58(3):862-70. doi: 10.1016/j.ijrobp.2003.09.012 [Crossref] [ Google Scholar]
- Fernandez-Palomo C, Schültke E, Smith R, Bräuer-Krisch E, Laissue J, Schroll C. Bystander effects in tumor-free and tumor-bearing rat brains following irradiation by synchrotron X-rays. Int J Radiat Biol 2013; 89(6):445-53. doi: 10.3109/09553002.2013.766770 [Crossref] [ Google Scholar]