Arch Iran Med. 25(4):257-266.
doi: 10.34172/aim.2022.42
Systematic Review
A Comprehensive Survey of the Relationship between Helicobacter pylori Infection and Atherosclerosis in the Iranian Population: A Systematic Review and Meta-analysis
Masoud Keikha 1, 2  , Mohsen Karbalaei 3, *
, Mohsen Karbalaei 3, * 
Author information:
1Antimicrobial Resistance Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
2Department of Microbiology and Virology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
3Department of Microbiology and Virology, School of Medicine, Jiroft University of Medical Sciences, Jiroft, Iran
*
Corresponding Author: Mohsen Karbalaei, PhD; Department of Microbiology and Virology, School of Medicine, Jiroft University of Medical Sciences, Jiroft, Iran. Tel:+989131933612; Email:
mohsen.karbalaei@jmu.ac.ir
Abstract
Background:
Helicobacter pylori is a gram-negative, spiral-shaped, and microaerophilic bacterium that inhabits the human gastric mucosa and is considered to be the most important etiologic agent for gastrointestinal disorders. Recently, however, there is ample evidence to suggest an association between H. pylori infection and extragastric complications, particularly atherosclerosis. The aim of this study was to evaluate the rate of H. pylori infection and the risk of atherosclerosis in an Iranian population.
Methods:
We conducted a comprehensive electronic search on PubMed, Scopus, Google scholar, IranMedex, SID, ISC, and Magiran to find the main published documents related to the relationship between H. pylori and atherosclerosis in Iran. A summary odds ratio with 95% confidence interval was used to investigate the potential association between H. pylori and atherosclerosis. In addition, the heterogeneity between studies was assessed by the I 2 index and the Cochrane Q-test. Publication bias was determined using a funnel plot.
Results:
A total of 12 studies met our inclusion criteria and were included in the present study. The results showed that there is a significant positive relationship between infection with this bacterium and the two-fold risk of developing atherosclerosis in the Iranian population (OR: 1.44; 95% CI: 1.07–1.95). However, the heterogeneity was significant and we observed a slight publication bias.
Conclusion:
We confirmed a positive relationship between H. pylori infection and atherosclerosis in the Iranian population, which is similar to other reports from Western countries. Most likely, H. pylori infection can increase the risk of developing atherosclerosis.
Keywords: Atherosclerosis, Cardiovascular disease, Helicobacter pylori, Iran
Copyright and License Information
© 2022 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Keikha M, Karbalaei M. A comprehensive survey of the relationship between helicobacter pylori infection and atherosclerosis in the iranian population: a systematic review and meta-analysis. Arch Iran Med. 2022;25(4):257-266. doi: 10.34172/aim.2022.42
Introduction
Cardiovascular diseases (CVDs) are the most common cause of death worldwide (50% of global mortality). With an unprecedented increase in mortality over the past two decades, CVD has become one of the major concerns of the global health systems.1-3 Atherosclerosis is undoubtedly the most important predisposing factor for cardiovascular disorders with a high prevalence in the developed as well as the developing countries.2-5 Atherosclerosis is a chronic disease and several factors are involved in its development.6 It should be noted that traditional risk factors such as smoking, obesity, hypertension, hypercholesterolemia, and host genome polymorphisms do not have a significant effect on the pathogenesis of atherosclerosis, and recent studies show that inflammatory diseases play a major role in this regard.7,8 Infectious agents are considered as the most important inflammatory triggers in the body, as well as a risk factor for atherosclerosis due to stimulating the inflammatory process and damaging vascular endothelial cells.9,10 According to the literature, the most important infectious agents that are associated with atherosclerosis include: Chlamydia pneumoniae, Mycobacterium tuberculosis, Helicobacter pylori, hepatitis C virus,human immunodeficiency virus,Epstein-Barr virus,hepatitis B virus,human T lymphotropic virus type I, andcytomegalovirus.11-18 Helicobacter pylori is a gram-negative, microaerophilic, and helical bacterium found in the lining of the human stomach and is the etiologic cause of chronic gastritis, gastric ulcer, duodenal ulcer, and gastric cancer.19,20 Recent studies have shown that H. pylori is also isolated from dental plaques, human saliva, duodenum, feces and atherosclerotic plaques, and is strongly associated with the extragastrointestinal disorders such as idiopathic thrombocytopenic purpura, neurological disorders (stroke events), psychiatric, gynecological, pre-eclampsia, infertility, glaucoma, dermatologic complications, lung cancer, iron deficiency anemia, autoimmune diseases, and atherosclerosis.21-33 Unfortunately, the rate of colonization with H. pylori is high worldwide, especially in Asia (from 25%–50% in developed countries to 90% in developing countries); the eradication of H. pylori infection has decreased in recent years due to problems such as elimination of antibiotics in acidic gastric conditions, bacterial infiltration under the gastric mucosa, and antibiotic resistance.34-36 According to studies, the rate of gastric cancer in Asia is higher than Western countries, and many researchers attribute this to the high colonization with H. pylori in this geographical area.37-39 Moreover, the rate of atherosclerosis in developing countries is higher than developed countries.4,40,41 The hypothesis regarding the correlation between H. pylori and atherosclerosis was first proposed by Mendall et al in 1994.42 Based on extensive studies on the correlation of infection with this bacterium and atherosclerosis, it seems that one of the most important reasons for the increase in atherosclerosis in developing countries could be the high prevalence of colonization with H. pylori in these regions (for example, bacteria isolated from atherosclerotic plaques of the patients).43-45 However, some sources have ruled out the association between H. pylori and atherosclerosis and there is no exact answer to this question, so more studies are needed.46-48 The rate of atherosclerosis and cardiovascular disorders is high in Iran and according to the Iranian Society of Atherosclerosis statement, 300 deaths occur due to CVDs in Iran every day. In addition, due to the high rate of colonization with H. pylori in Iran (estimated at more than 85%), this country is recognized as one of the most suitable places to investigate the possible link between infection with this bacterium and atherosclerosis. The present study was conducted to investigate the relationship between colonization with this bacterium and susceptibility to atherosclerosis in Iranian patients.
Materials and Methods
Search Strategy
The present comprehensive meta-analysis was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline proposed by Liberati et al.49 First, we conducted a fully electronic computer search to retrieve all relevant studies to assess the relationship between colonization with H. pylori and atherosclerosis progression. For this purpose, a systematic search was performed using online databases including PubMed, Scopus, Google scholar, as well as national databanks such as IranMedex, SID, ISC and Magiran regardless of publication date and language restrictions by the end of 2019. The search terms were: “Helicobacter pylori”, “Iran”, “Atherosclerosis”, “Cardiovascular diseases”, and “Coronary artery disease”.
Selection Criteria
To find eligible studies, the title, introduction, and full text of potentially collected articles were carefully screened independently by the two authors. The inclusion criteria for the selected articles included: I) studies related to H. pylori infection in atherosclerotic plaques, II) studies performed in an Iranian population, III) studies published in English or Persian, IV) original studies including cross-sectional, case-control and longitudinal studies, V) articles examining infection by standard methods such as conventional microbiology, PCR, and ELISA, VI) human experiments, VII) studies on clinical specimens, and VIII) full text availability. The exclusion criteria were: 1) in-vitro studies and non-human investigation, 2) studies containing insufficient results, 3) case reports and review articles, 4) studies evaluating co-infection and other infectious agents other than H. pylori, 5) duplicate articles, and 6) studies with abstract only. In addition, the bibliography of the articles was manually searched to prevent loss of related articles.
Quality Assessment and Data Extraction
The Joanna Briggs Institute (JBI) critical appraisal checklist was used to evaluate the quality of the eligible studies. The required data included, first author, publication year, location of studies, frequency of H. pylori infection in both case and control groups, diagnostic methods, and references number (Table 1).
Table 1.
Characteristics of the Iranian Studies About Frequency of H. pylori in CAD Patients
|
First Author
|
Year
|
Provinces
|
Type of Study
|
Atherosclerosis Plaque
|
Healthy Individuals
|
Diagnostic Method
|
Ref.
|
|
No. HP Positive
|
Total
|
N (%)
|
No. HP Positive
|
Total
|
N (%)
|
| Sadeghian et al |
2019 |
Mashhad |
Cross-sectional |
0 |
30 |
0 |
0 |
30 |
0 |
PCR |
22
|
| Abibiglou et al |
2018 |
Tabriz |
Cross-sectional |
1 |
28 |
3.57 |
NA |
NA |
NA |
PCR |
50
|
| Izadi et al |
2012 |
Tehran |
Cross-sectional |
56 |
105 |
53.33 |
NA |
NA |
NA |
PCR/serology |
51
|
| Gharehdaghi et al |
2018 |
Tehran |
Case-control |
10 |
90 |
11.11 |
0 |
90 |
0 |
PCR |
52
|
| Nouzari et al |
2009 |
Tehran |
Case-control |
56 |
70 |
80 |
38 |
60 |
65 |
Serology |
53
|
| Yazdi et al |
2014 |
Tehran |
Cross-sectional |
1 |
90 |
1.11 |
NA |
NA |
NA |
Culture |
54
|
| Pouria et al |
2009 |
Kermanshah |
Case-control |
8 |
30 |
26.66 |
3 |
30 |
10 |
Serology |
55
|
| Vafaeimanesh et al |
2014 |
Qom |
Case-control |
47 |
62 |
75.80 |
10 |
20 |
50 |
Serology |
56
|
| Sayyah et al |
2012 |
Qazvin |
Case-control |
32 |
40 |
68.08 |
18 |
40 |
45 |
Serology |
57
|
| Ansari et al |
2010 |
Urmia |
Case-control |
49 |
100 |
49 |
56 |
89 |
49.5 |
Serology |
58
|
| Davoudi et al |
2010 |
Tehran |
Case-control |
40 |
69 |
57.97 |
48 |
84 |
57.1 |
Serology |
59
|
| Ashtari et al |
2006 |
Isfahan |
Case-control |
29 |
42 |
69.04 |
29 |
43 |
67.4 |
Serology |
60
|
Data Analysis
The possible relationship between H. pylori infection and atherosclerosis was investigated using odds ratio (OR) with 95% confidence intervals (CIs). We used a random-effects model when the heterogeneity was high; heterogeneity was assessed using I2 statistic and Cochrane Q statistic (I2 > 25% and P value < 0.1). The statistical analysis for this study was done using the Comprehensive Meta-Analysis (CMA) software ver. 2.2 (Biostat, Englewood, NJ).61 Furthermore, the presence of publication bias was also determined by funnel plot asymmetry, Begg’s P value, and Egger’s P value tests.
Results
A total of 94 articles were collected throughout searching in global databases. The titles and abstracts of the studies were screened and the duplicate studies were excluded. Next, 42 studies were excluded in the screening process because they were unrelated to our original idea. Finally, 12articles that met our inclusion criteria were used for the current systematic review and meta-analysis (Figure 1). Of the 12 studies, eight were case-control and four were cross-sectional; all studies were conducted during 2006–2019. Five studies were related to Tehran, and one study had been done in cities such as Tabriz, Mashhad, Kermanshah, Qom, Qazvin, Urmia and Isfahan. In these studies, the information of 1242 participants comprising 756 patients with atherosclerosis and 486 healthy individuals were assessed. Of these, 43.8% were female and 56.2% were male. In addition, the mean age in the case and control groups was 62.7% and 57.4%, respectively.22,52,59,60 In four studies, the association between atherosclerosis and H. pylori infection was conflicting. In several studies, this relationship was 0–80%.22,53 However, none of the selected studies examined the association between eradication of H. pylori infection (or cagA gene status) and atherosclerosis.
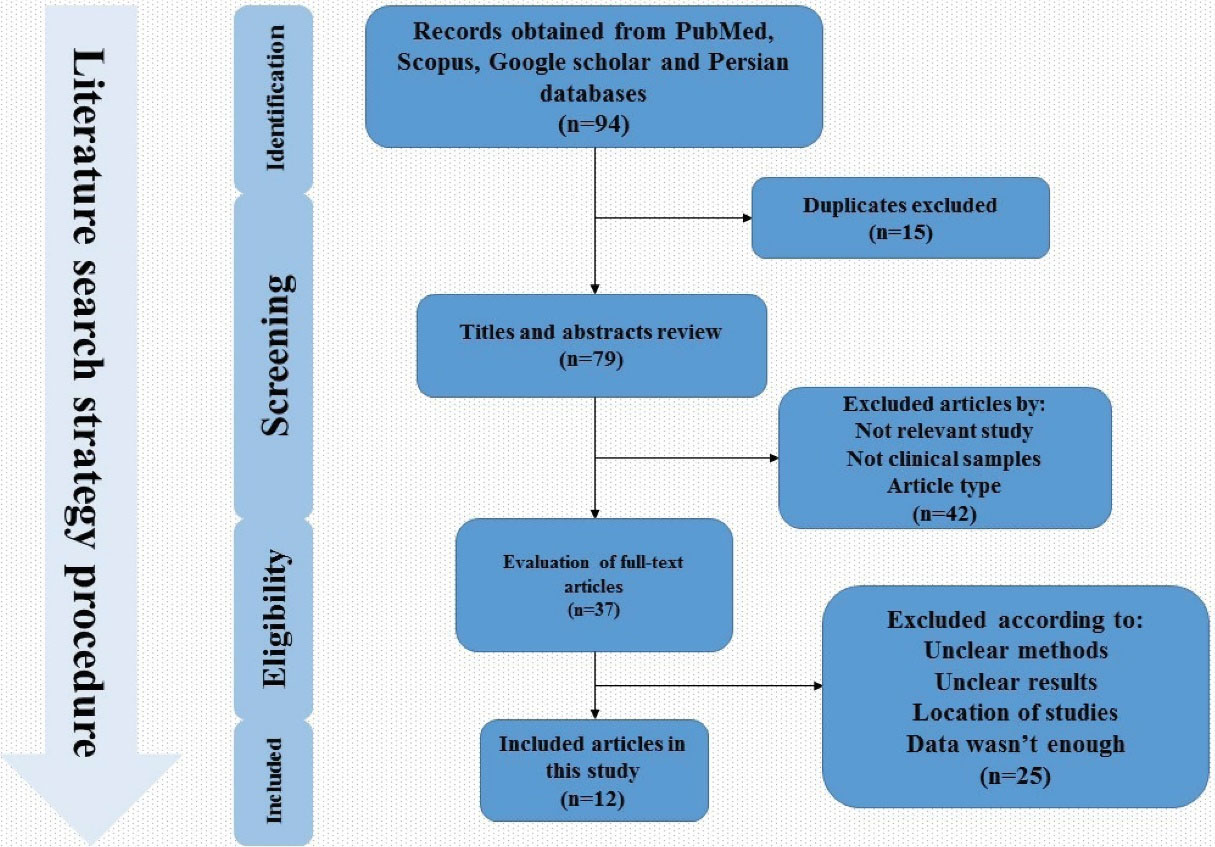
Figure 1.
Flowchart of Included Articles According to PRISMA Strategy.
.
Flowchart of Included Articles According to PRISMA Strategy.
We entered data from 1242 cases in the current meta-analysis; OR is more reliable than relative risk (RR) for predicting risk factors and clinical outcomes, especially in low sample size studies such as the present study.62,63 In general, the prevalence of H. pylori in atherosclerotic plaques in patients with coronary artery disease (CAD) was estimated at 55.6% (50.3–60.9 with 95% CIs; P value: 0.01). On the other hand, the rate of H. pylori infection in the control group was measured about 53.7% (49.3–58.1 with 95% CIs; P value: 0.01). According to studies, the rate of colonization with this bacterium is high in the population of Iran and we also showed high H. pylori infection in both case and control groups, which confirms previous epidemiological studies. Therefore, in case-control studies, the rate of H. pylori infection in atherosclerosis cases was significantly higher than normal individuals (P value = 0.002). We found a strong positive association between H. pylori infection and atherosclerosis in the Iranian population (OR: 1.44; 95% CIs: 1.07–1.95; P value: 0.01; I2: 67.66; Q-value: 34.02; P value: 0.01; Egger’s P value: 0.02; Begg’s P value: 0.18) (Figure 2). In addition, due to the significant heterogeneity between studies, we performed subgroup analysis based on the type of study to reduce potential heterogeneity between eligible studies. Based on the results of subgrouping analysis on both case-control (OR: 1.38; 95% CIs: 1.01–1.87; P value: 0.03; I2: 72.50; Q-value: 25.45; P value: 0.01; Egger’s P value: 0.02, Begg’s P value: 0.06) and cross-sectional (OR: 4.72; 95% CIs: 0.98–22.64; P value: 0.05; I2: 52.27; Q-value: 6.28; P value: 0.09; Egger’s P value: 0.08; Begg’s P value: 0.36) studies, we suggest that H. pylori infection can increase the risk of atherosclerosis in the Iranian patients. Also, funnel plot asymmetry showed a slight publication bias in the present study (Figure 3).
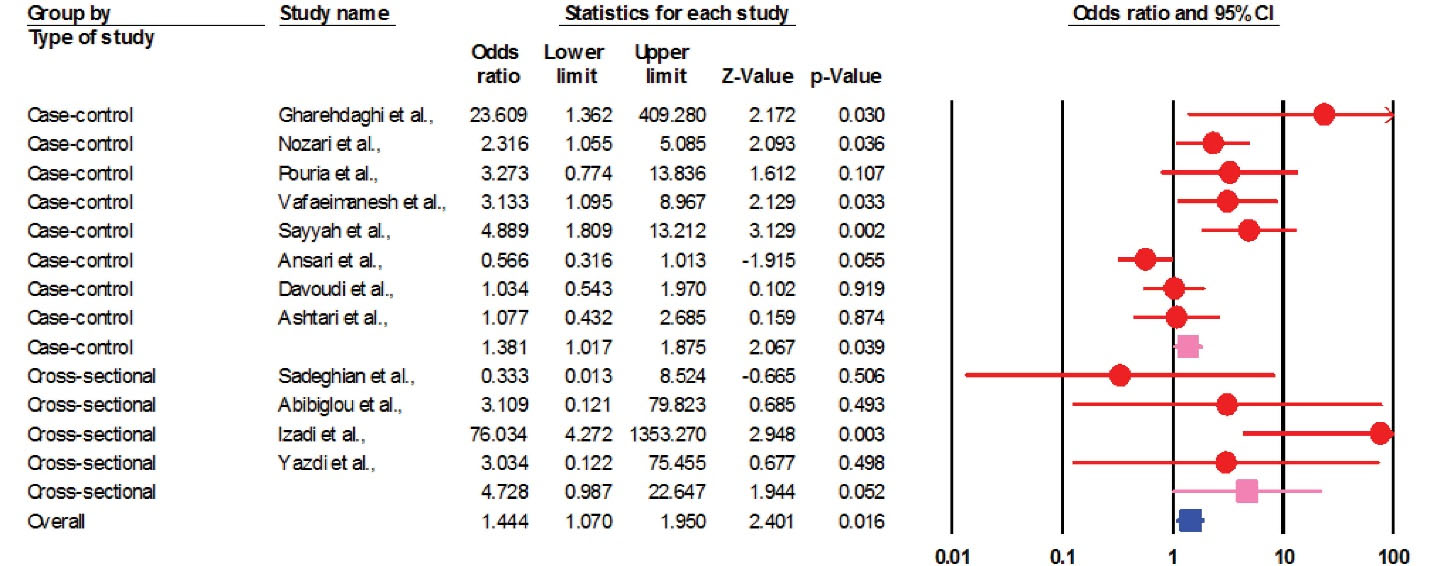
Figure 2.
Forest Plot of Pooled ORs of Association between H. pylori Infection and Atherosclerosis in the Iranian Population According to Type of Included Studies.
.
Forest Plot of Pooled ORs of Association between H. pylori Infection and Atherosclerosis in the Iranian Population According to Type of Included Studies.
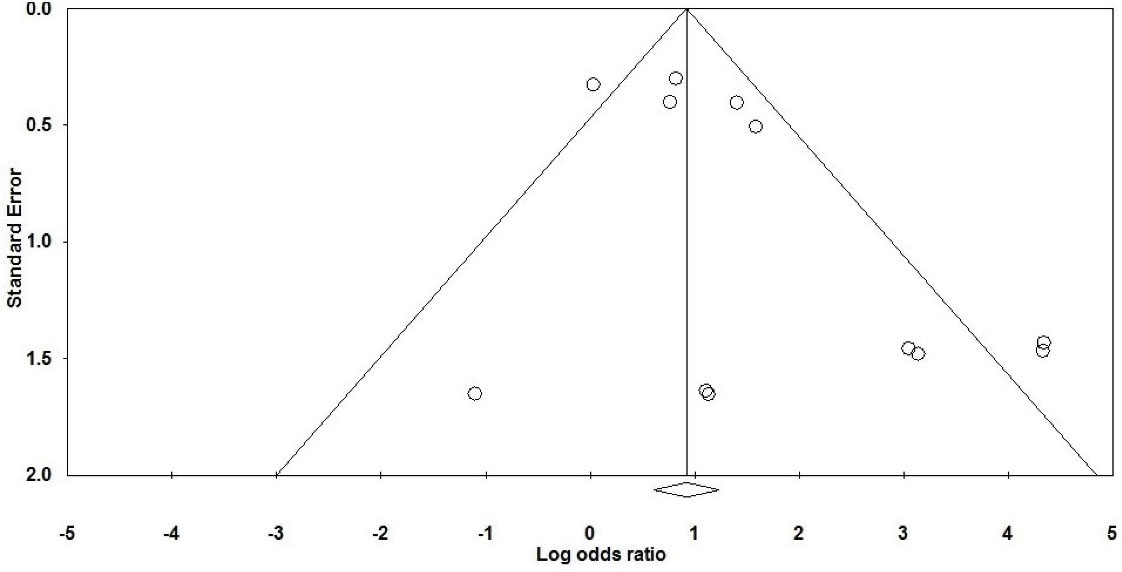
Figure 3.
Funnel Plot for Assessment of Publication Bias Which Represent Slight Significant Publication Bias.
.
Funnel Plot for Assessment of Publication Bias Which Represent Slight Significant Publication Bias.
Discussion
Atherosclerosis is the most common cause of CVDs, especially ischemic heart disease and stroke, and is among the top four causes of death worldwide.64 Atherosclerosis is a compound Greek word from athero meaning gruel or paste and sclerosis meaning hardness. It is a large and medium vascular disease that begins with damage to vascular endothelial cells and changes in blood circulation, followed by the formation of atherosclerotic plaques. The components of plaques include the necrotic cores, calcified regions, lipid particles, smooth muscle cells, endothelial cells, polymorphonuclear cells and foamy cells (alternative macrophages).64,65 Vascular endothelium damage (especially intima) and chronic inflammation are the most prominent stimuli for the formation of atherosclerotic plaques.64-66 Pathogens such as C. pneumoniae, M. tuberculosis and H. pylori, are among the most important infectious bacteria that can cause chronic inflammation to escape the immune system and appear to play a role in the formation of atherosclerotic plaques.22 After decades of early detection, it is now proven that traces of these bacteria are present in coronary, carotid and aortic atherosclerotic plaques.42,67 Bahrmand et al showed that C. pneumoniae infection is also associated with atherosclerosis; in that study, using PCR, the presence of this bacterium in arterial specimens with severe and mild lesions was 17% and 3%, respectively.18 In particular, eradication of H. pylori infection reduces C-reactive protein and proinflammatory response and improves endothelial dysfunction, and has a protective effect in the early stages of atherosclerosis.68-70 According to this meta-analysis, the rate of colonization with this pathogen is significantly associated with the formation of atherosclerotic plaques in Iranian patients with CAD, and may predispose individuals to cardiovascular disorders (Figure 4).
Studies have shown that chronic inflammation (such as H. pylori infection) during atherosclerosis stimulates the activation of Th1 cells and production of proinflammatory cytokines (IL-1, IL-6, IFN-γ and TNF-α), which in turn leads to the employment of inflammatory cells, especially polymorphonuclear cells. Macrophages also enter into the location in response to MCP1/2, and then induce the inflammatory reactions that lead to the destruction and dysfunction of the endothelial cells.65-67,71,72 By stopping the production of stomach acid, this pathogen can lead to atrophy and malabsorption of vitamin B12 and folic acid, resulting in high levels of homocysteine, which stimulates the production of blood nitric oxide by damaging the vessel wall.73-76 Recent studies have shown that people infected with H. pylori may develop dyslipidemia.77 Hoffmeister et al showed that the CAD patients infected with H. pylori had higher levels of cholesterol, low-density lipoprotein (LDL), triglyceride, and apolipoprotein-B compared to the CAD patients infected with C. pneumonia or cytomegalovirus; high-density lipoprotein (HDL) levels of these patients was also lower.78 In contrast, in recovered individuals, serum levels of HDL and apolipoprotein-AI/AII increase, while cholesterol, triglyceride, and LDL levels decrease.77-79 Metabolic disorders can also cause atherosclerosis.80 Gillum et al showed that there is a significant relationship between H. pylori seropositivity and CAD in diabetic patients.81 In addition, de Luis showed that CAD and cerebrovascular disorders are high in diabetic patients infected with H. pylori.82 Polyzos et al found that infection with H. pylori is associated with resistance to insulin, and that H. pylori infection may lead to atherosclerosis by affecting glucose metabolism, such that glucose resistance improves subsequent to the eradication of the infection while adiponectin (a factor for preventing metabolic disorders) levels increase.83 Disorders such as high blood pressure and arterial stiffness play a role in the process of atherosclerosis, and studies have shown that there is a significant relationship between H. pylori infection and these complications.84,85 Recently, the effect of virulence factors on atherosclerosis has been studied and the previous reports showed a significant relationship between infection with cagA-positive strains and carotid plaque.86-88 Also, De Bastiani et al showed that CagA seropositivity is very high in patients with stroke.89 In this regard, cagA-positive strains have been shown to induce atherosclerosis with effects such as vascular endothelial cell destruction, oxidized LDL modification, and inflammatory stimulation; eradication of these strains stops these injuries and has a protective effect in patients.90-92 In addition, it has been now suggested that CagA and heat shock proteins stimulate autoantibody production and endothelial dysfunction, which ultimately leads to atherosclerosis.93,94 To assess cardiovascular risk factors, Longo-Mbenza et al followed 205 patients for 10 years in their study and found that H. pylori IgG titer was significantly higher in the population of CAD patients.95 Following their study on 2029 patients in South Korea, Park et al found that there was a significant relationship between H. pylori positivity and CAD patients (OR: 1.23; P = 0.049).96 Other case-control studies conducted in India, Turkey, and Japan confirmed the results of previous studies.96-99 Our study also confirms the relationship between H. pylori infection and atherosclerosis in CAD patients. Bacteria isolated from atherosclerotic plaques constitute one of the most important pieces of evidence to confirm the role of this microorganism in atherosclerosis and CAD.67,94 So far, extensive studies have been conducted in this area; for example, the rate of H. pylori isolation from atherosclerosis in the study by Jha et al was 27.2–33.5%.97 In another study in Argentina, the rate of isolated bacterium from carotid plaque was about 83%.100 In a cross-sectional study in Turkey, Kilic et al found that the isolation of H. pylori from atherosclerotic plaques and non-atherosclerotic vascular wall samples was 48.2% and 19.2%, respectively.101 However, H. pylori has not been isolated from atherosclerotic plaques in studies conducted in Italy and Poland.102,103 Rahmani et al demonstrated in their meta-analysis that there was a significant relationship between the infection with H. pylori and myocardial infarction in the Iranian patients (OR: 2.53).104 In a meta-analysis of 18 epidemiological studies, Danesh et al found no significant relationship between H. pylori infection and coronary heart disease.105 In a meta-analysis performed by Wang et al on a 4041 of stroke patients, there was a significant relationship between H. pylori infection and ischemic stroke.23 In another study by Yu et al, a significant relationship was observed between H. pylori infection and CAD in the European (OR: 2.11) and American (OR: 1.43) patients.106 However, in another meta-analysis, 13 studies were reviewed and no significant relationship was found between H. pylori infection and stroke.107 Because recent studies have suggested that infection with strains lacking CagA and VacA may lead to controversial results, the role of virulence factors in CVDs has received more attention.67,94 In a study on 684 CAD patients, Mayr et al found that infection with cagA-positive strains was significantly associated with atherosclerosis (P = 0.08).108 In a cross-sectional study on seven case-control studies, Cremonini et al found a significant relationship between stroke and CagA seropositive strains (OR: 1.65).109 In addition, Sun et al showed that there was no relationship between cagA-positive strains infection and coronary heart disease (OR: 0.8).110 Our study had several limitations including (I) small number of studies, (II) in some studies, diagnosis was based on the evaluation of H. pylori IgG seropositivity, but this test also yields false positive results after treatment, (III) studies were restricted to geographic regions and this led to controversial results, (IV) the type of CAD was not considered in several studies (e.g. coronary disease, ischemia, stroke, etc.), (V) virulence factors such as CagA and VacAs1m1 were not investigated in all included studies, (IV) heterogeneity was significant, (VII) presence of publication bias for the studies, (VIII) lack of subgroup analysis due to lack of access to raw data. Therefore, the results of the study must be carefully interpreted. Further research is needed with more participants to confirm the validity of the current findings.
In conclusion, in the present study, we statistically investigated the effect of H. pylori infection in development of atherosclerosis in the Iranian population. Our study shows that the rate of H. pylori infection in cardiovascular patients is higher compared to healthy individuals. We found a strong positive association between H. pylori infection and susceptibility to atherosclerosis risk. Our results are consistent with previous meta-analyses in the populations of Western countries. Most likely, H. pylori infection can increase the risk of atherosclerosis by inducting proinflammatory response, lymphogenesis, platelet activation, intima media thickness, and endothelial dysfunction. Based on the available findings, H. pylori infection can be considered as a risk factor for CVDs, especially atherosclerosis.
Acknowledgements
We appreciate from both Mashhad University of Medical Sciences and Jiroft University of Medical Sciences.
Authors’ Contribution
MaK contributed to design of the work and analysis of data. MoK drafted the work and substantively revised it. Both authors read and approved the final manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Conflict of Interest Disclosures
There is no any conflict of interest among the all authors.
Ethical Statement
Not applicable (this paper was provided based on researching in global databases).
Funding
We have not received any funding for this research.
References
- Kumar V, Abbas A, Fausto N, Aster J. The Gastrointestinal Tract. Robbins and Cotran Pathologic Basis of Disease, Professional. 8th ed. Philadelphia: Saunders; 2010.
- Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018; 138(11):1100-12. doi: 10.1161/circulationaha.117.033369 [Crossref] [ Google Scholar]
- Lee KK, Stelzle D, Bing R, Anwar M, Strachan F, Bashir S. Global burden of atherosclerotic cardiovascular disease in people with hepatitis C virus infection: a systematic review, meta-analysis, and modelling study. Lancet Gastroenterol Hepatol 2019; 4(10):794-804. doi: 10.1016/s2468-1253(19)30227-4 [Crossref] [ Google Scholar]
- Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y. Cardiovascular disease and risk factors in Asia: a selected review. Circulation 2008; 118(25):2702-9. doi: 10.1161/circulationaha.108.790048 [Crossref] [ Google Scholar]
- Moradi M, Varasteh E. Coronary atherosclerosis evaluation among Iranian patients with zero coronary calcium score in computed tomography coronary angiography. Adv Biomed Res 2016; 5:24. doi: 10.4103/2277-9175.175920 [Crossref] [ Google Scholar]
- Jacotot B. Atherosclerosis, a multifactor lesion justifying multirisk care. Atherosclerosis 1994; 110 Suppl:S1-2. doi: 10.1016/0021-9150(94)05370-x [Crossref] [ Google Scholar]
- Al-Obeidy ES, Saeed BN. H. pylori Infection in Iraqi patients with ischemic heart diseases. J Fac Med Baghdad 2011; 53(1):29-31. doi: 10.32007/jfacmedbagdad.531904 [Crossref] [ Google Scholar]
- Fong IW. Emerging relations between infectious diseases and coronary artery disease and atherosclerosis. CMAJ 2000; 163(1):49-56. [ Google Scholar]
- Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 2006; 6(7):508-19. doi: 10.1038/nri1882 [Crossref] [ Google Scholar]
- Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol 2017; 14(2):79-87. doi: 10.1038/nrcardio.2016.183 [Crossref] [ Google Scholar]
- Jha HC, Srivastava P, Divya A, Prasad J, Mittal A. Prevalence of Chlamydophila pneumoniae is higher in aorta and coronary artery than in carotid artery of coronary artery disease patients. APMIS 2009; 117(12):905-11. doi: 10.1111/j.1600-0463.2009.02553.x [Crossref] [ Google Scholar]
- Tien PC, Schneider MF, Cole SR, Cohen MH, Glesby MJ, Lazar J. Association of hepatitis C virus and HIV infection with subclinical atherosclerosis in the women’s interagency HIV study. AIDS 2009; 23(13):1781-4. doi: 10.1097/QAD.0b013e32832d7aa8 [Crossref] [ Google Scholar]
- Ghotaslou R, Aslanabadi N, Ghojazadeh M. Hepatitis B virus infection and the risk of coronary atherosclerosis. Ann Acad Med Singap 2008; 37(11):913-5. [ Google Scholar]
- Müller BT, Huber R, Henrich B, Adams O, Berns G, Siebler M. Chlamydia pneumoniae, herpes simplex virus and cytomegalovirus in symptomatic and asymptomatic high-grade internal carotid artery stenosis. Does infection influence plaque stability? Vasa 2005; 34(3):163-9. doi: 10.1024/0301-1526.34.3.163 [Crossref] [ Google Scholar]
- Apostolou F, Gazi IF, Lagos K, Tellis CC, Tselepis AD, Liberopoulos EN. Acute infection with Epstein-Barr virus is associated with atherogenic lipid changes. Atherosclerosis 2010; 212(2):607-13. doi: 10.1016/j.atherosclerosis.2010.06.006 [Crossref] [ Google Scholar]
- Rota S, Rota S. Mycobacterium tuberculosis complex in atherosclerosis. Acta Med Okayama 2005; 59(6):247-51. doi: 10.18926/amo/31958 [Crossref] [ Google Scholar]
- Ayada K, Yokota K, Hirai K, Fujimoto K, Kobayashi K, Ogawa H. Regulation of cellular immunity prevents Helicobacter pylori-induced atherosclerosis. Lupus 2009; 18(13):1154-68. doi: 10.1177/0961203309106600 [Crossref] [ Google Scholar]
- Bahrmand AR, Bahadori M, Hossaini A, Velayati AA, Aghabozorgy S, Shakoor A. Chlamydia pneumoniae DNA is more frequent in advanced than in mild atherosclerosis lesions. Scand J Infect Dis 2004; 36(2):119-23. doi: 10.1080/00365540310018888 [Crossref] [ Google Scholar]
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002; 347(15):1175-86. doi: 10.1056/NEJMra020542 [Crossref] [ Google Scholar]
- Keikha M, Ali-Hassanzadeh M, Karbalaei M. Association of Helicobacter pylori vacA genotypes and peptic ulcer in Iranian population: a systematic review and meta-analysis. BMC Gastroenterol 2020; 20(1):266. doi: 10.1186/s12876-020-01406-9 [Crossref] [ Google Scholar]
- Kim N, Lim SH, Lee KH, You JY, Kim JM, Lee NR. Helicobacter pylori in dental plaque and saliva. Korean J Intern Med 2000; 15(3):187-94. doi: 10.3904/kjim.2000.15.3.187 [Crossref] [ Google Scholar]
- Sadeghian MH, Tabatabaee Yazdi SA, Ayatollahi H, Ghazvini K, Keramati MR, Ghayoor Karimiani E. Absence of Helicobacter pylori infection in coronary atherosclerosis disease in Northeast of Iran. Artery Res 2013; 7(3-4):211-5. doi: 10.1016/j.artres.2013.08.003 [Crossref] [ Google Scholar]
- Wang ZW, Li Y, Huang LY, Guan QK, Xu DW, Zhou WK. Helicobacter pylori infection contributes to high risk of ischemic stroke: evidence from a meta-analysis. J Neurol 2012; 259(12):2527-37. doi: 10.1007/s00415-012-6558-7 [Crossref] [ Google Scholar]
- Roubaud Baudron C, Letenneur L, Langlais A, Buissonnière A, Mégraud F, Dartigues JF. Does Helicobacter pylori infection increase incidence of dementia? The Personnes Agées QUID Study. J Am Geriatr Soc 2013; 61(1):74-8. doi: 10.1111/jgs.12065 [Crossref] [ Google Scholar]
- Shaban MM, Kandil HO, Elshafei AH. Helicobacter pylori seropositivity in patients with hyperemesis gravidarum. Am J Med Sci 2014; 347(2):101-5. doi: 10.1097/MAJ.0b013e31827bef91 [Crossref] [ Google Scholar]
- Franceschi F, Di Simone N, D’Ippolito S, Castellani R, Di Nicuolo F, Gasbarrini G. Antibodies anti-CagA cross-react with trophoblast cells: a risk factor for pre-eclampsia?. Helicobacter 2012; 17(6):426-34. doi: 10.1111/j.1523-5378.2012.00966.x [Crossref] [ Google Scholar]
- Ambrosini G, Andrisani A, Fiore C, Faggian D, D’Antona D, Ragazzi E. Anti-Helicobacter pylori antibodies in cervical mucus: a new cause of infertility. Eur J Obstet Gynecol Reprod Biol 2011; 155(2):157-60. doi: 10.1016/j.ejogrb.2010.12.001 [Crossref] [ Google Scholar]
- Bagnis A, Izzotti A, Saccà SC. Helicobacter pylori, oxidative stress and glaucoma. Dig Liver Dis 2012; 44(11):963-4. doi: 10.1016/j.dld.2012.05.009 [Crossref] [ Google Scholar]
- Magen E, Mishal J. Possible benefit from treatment of Helicobacter pylori in antihistamine-resistant chronic urticaria. Clin Exp Dermatol 2013; 38(1):7-12. doi: 10.1111/j.1365-2230.2012.04467.x [Crossref] [ Google Scholar]
- Deng B, Li Y, Zhang Y, Bai L, Yang P. Helicobacter pylori infection and lung cancer: a review of an emerging hypothesis. Carcinogenesis 2013; 34(6):1189-95. doi: 10.1093/carcin/bgt114 [Crossref] [ Google Scholar]
- Papagiannakis P, Michalopoulos C, Papalexi F, Dalampoura D, Diamantidis MD. The role of Helicobacter pylori infection in hematological disorders. Eur J Intern Med 2013; 24(8):685-90. doi: 10.1016/j.ejim.2013.02.011 [Crossref] [ Google Scholar]
- Payandeh M, Sohrabi N, Zare ME, Nasir Kansestani A, Hashemian AH. Platelet count response to Helicobacter pylori eradication in Iranian patients with idiopathic thrombocytopenic purpura. Mediterr J Hematol Infect Dis 2012; 4(1):e2012056. doi: 10.4084/mjhid.2012.056 [Crossref] [ Google Scholar]
- Youssefi M, Tafaghodi M, Farsiani H, Ghazvini K, Keikha M. Helicobacter pylori infection and autoimmune diseases; Is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? A systematic review and meta-analysis study. J Microbiol Immunol Infect 2021; 54(3):359-69. doi: 10.1016/j.jmii.2020.08.011 [Crossref] [ Google Scholar]
- Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev 1997; 10(4):720-41. doi: 10.1128/cmr.10.4.720 [Crossref] [ Google Scholar]
- Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 2006; 19(3):449-90. doi: 10.1128/cmr.00054-05 [Crossref] [ Google Scholar]
- Bazzoli F, Pozzato P, Rokkas T. Helicobacter pylori: the challenge in therapy. Helicobacter 2002; 7 Suppl 1:43-9. doi: 10.1046/j.1523-5378.7.s1.7.x [Crossref] [ Google Scholar]
- Singh K, Ghoshal UC. Causal role of Helicobacter pylori infection in gastric cancer: an Asian enigma. World J Gastroenterol 2006; 12(9):1346-51. doi: 10.3748/wjg.v12.i9.1346 [Crossref] [ Google Scholar]
- Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med 2008; 47(12):1077-83. doi: 10.2169/internalmedicine.47.0975 [Crossref] [ Google Scholar]
- Yousefi B, Mohammadlou M, Abdollahi M, Salek Farrokhi A, Karbalaei M, Keikha M. Epigenetic changes in gastric cancer induction by Helicobacter pylori. J Cell Physiol 2019; 234(12):21770-84. doi: 10.1002/jcp.28925 [Crossref] [ Google Scholar]
- Wong MC, Zhang DX, Wang HH. Rapid emergence of atherosclerosis in Asia: a systematic review of coronary atherosclerotic heart disease epidemiology and implications for prevention and control strategies. Curr Opin Lipidol 2015; 26(4):257-69. doi: 10.1097/mol.0000000000000191 [Crossref] [ Google Scholar]
- Ansari R, Khosravi A, Bahonar A, Shirani S, Kelishadi R, Khosravi Z. Risk factors of atherosclerosis in male smokers, passive smokers, and hypertensive nonsmokers in central Iran. ARYA Atheroscler 2012; 8(2):90-5. [ Google Scholar]
- Mendall MA, Goggin PM, Molineaux N, Levy J, Toosy T, Strachan D. Relation of Helicobacter pylori infection and coronary heart disease. Br Heart J 1994; 71(5):437-9. doi: 10.1136/hrt.71.5.437 [Crossref] [ Google Scholar]
- Chmiela M, Gajewski A, Rudnicka K. Helicobacter pylori vs coronary heart disease - searching for connections. World J Cardiol 2015; 7(4):187-203. doi: 10.4330/wjc.v7.i4.187 [Crossref] [ Google Scholar]
- He C, Yang Z, Lu NH. Helicobacter pylori-an infectious risk factor for atherosclerosis?. J Atheroscler Thromb 2014; 21(12):1229-42. doi: 10.5551/jat.25775 [Crossref] [ Google Scholar]
- Ameriso SF, Fridman EA, Leiguarda RC, Sevlever GE. Detection of Helicobacter pylori in human carotid atherosclerotic plaques. Stroke 2001; 32(2):385-91. doi: 10.1161/01.str.32.2.385 [Crossref] [ Google Scholar]
- Jia EZ, Zhao FJ, Hao B, Zhu TB, Wang LS, Chen B. Helicobacter pylori infection is associated with decreased serum levels of high density lipoprotein, but not with the severity of coronary atherosclerosis. Lipids Health Dis 2009; 8:59. doi: 10.1186/1476-511x-8-59 [Crossref] [ Google Scholar]
- Zhu J, Quyyumi AA, Muhlestein JB, Nieto FJ, Horne BD, Zalles-Ganley A. Lack of association of Helicobacter pylori infection with coronary artery disease and frequency of acute myocardial infarction or death. Am J Cardiol 2002; 89(2):155-8. doi: 10.1016/s0002-9149(01)02192-0 [Crossref] [ Google Scholar]
- Lee SY, Kim DK, Son HJ, Lee JH, Kim YH, Kim JJ. The impact of Helicobacter pylori infection on coronary heart disease in a Korean population. Korean J Gastroenterol 2004; 44(4):193-8. [ Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6(7):e1000100. doi: 10.1371/journal.pmed.1000100 [Crossref] [ Google Scholar]
- Abibiglou SS, Baghi HB, Memar MY, Safaei N, Parvizi R, Banani M. The presence of Porphyromonasgingivalis, Chlamydia pneumonia, Helicobacter pylori, Mycoplasma pneumonia and Enterobacter hormaechei DNA in the atherosclerotic plaques. J Res Med Dent Sci 2018; 6(3):1-6. [ Google Scholar]
- Izadi M, Fazel M, Sharubandi SH, Saadat SH, Moshkani Farahani M, Nasseri MH. Helicobacter species in the atherosclerotic plaques of patients with coronary artery disease. Cardiovasc Pathol 2012; 21(4):307-11. doi: 10.1016/j.carpath.2011.09.011 [Crossref] [ Google Scholar]
- Gharehdaghi J, Nasr R, Kheirvari Khezerloo J, Akbari Eidgahi MR, Tabasi M, Ghasemi M. Detection of H. pylori infection in atherosclerotic plaques of 180 corpses in referred to forensic medicine center of Tehran in 2016-2017. Iran J Public Health 2018; 47(11):1780-2. [ Google Scholar]
- Nouzari Y, Akiash N, Ebrahimi Daryani N. Association between Helicobacter pylori infection and atherosclerotic coronary artery disease. Iran J Pathol 2009; 4(1):1-4. [ Google Scholar]
- Karimi Yazdi MM, Lotfala Moradi S, Khiabani Rad P, Esmaeili Benvidi M, Eslami G, Taherpour A. Investigating the presence of Helicobacter pylori in atheroma plaques of arteries in patients with atherosclerosis. Iran J Public Health 2014; 43(2):189. [ Google Scholar]
- Pouria A, Masoumi M, Rafiei E, Sabzi F, Hossein ZH, Salehi M. Relationship between Chlamydia pneumonia and Helicobacter pylori with atherosclerosis. Yafteh 2008; 10(2):3-11. [ Google Scholar]
- Vafaeimanesh J, Parham M, Seyyedmajidi M, Bagherzadeh M. Helicobacter pylori infection and insulin resistance in diabetic and nondiabetic population. ScientificWorldJournal 2014; 2014:391250. doi: 10.1155/2014/391250 [Crossref] [ Google Scholar]
- Sayyah S, Akbarian M, Abdollah-Salimi S, Tavakoli M, Pahlevan AA. Coexistence of gastric Helicobacter pylori infection and coronary artery diseases in Qazvin (Iran). J Inflamm Dis 2012; 16(1):36-43. [ Google Scholar]
- Khadem Ansari MH, Rasmi Y, Manafi M, Rahimipour A, Ghadermarzi E. The evaluation of Helicobacter pylori infection and cardiovascular disease risk factors with atherosclerosis. Stud Med Sci 2010; 21(1):17-23. [ Google Scholar]
- Davoudi S, Omran A, Boroumand M, Rahimian N, Saadat S. Association between Helicobacter. pylori and coronary artery disease. Open Med 2011; 6(1):107-12. doi: 10.2478/s11536-010-0051-4 [Crossref] [ Google Scholar]
- Ashtari F, Shaigannezhad V, Saberi A, Rabiei E. Relationship between Helicobacter pylori immunoglobulin G antibody and thrombotic ischemic stroke. Acta Med Iran 2008; 46(4):303-6. [ Google Scholar]
- Karbalaei M, Keikha M. Potential association between the hopQ alleles of Helicobacter pylori and gastrointestinal diseases: a systematic review and meta-analysis. Meta Gene 2020; 26:100816. doi: 10.1016/j.mgene.2020.100816 [Crossref] [ Google Scholar]
- Schmidt CO, Kohlmann T. When to use the odds ratio or the relative risk?. Int J Public Health 2008; 53(3):165-7. doi: 10.1007/s00038-008-7068-3 [Crossref] [ Google Scholar]
- Richy F, Bruyere O, Ethgen O, Cucherat M, Henrotin Y, Reginster JY. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis: a comprehensive meta-analysis. Arch Intern Med 2003; 163(13):1514-22. doi: 10.1001/archinte.163.13.1514 [Crossref] [ Google Scholar]
- Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol 2017; 13(6):368-80. doi: 10.1038/nrneph.2017.51 [Crossref] [ Google Scholar]
- Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 2009; 27:165-97. doi: 10.1146/annurev.immunol.021908.132620 [Crossref] [ Google Scholar]
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS. Atherosclerosis. Nat Rev Dis Primers 2019; 5(1):56. doi: 10.1038/s41572-019-0106-z [Crossref] [ Google Scholar]
- Karbasi-Afshar R, Khedmat H, Izadi M. Helicobacter pylori Infection and atherosclerosis: a systematic review. Acta Med Iran 2015; 53(2):78-88. [ Google Scholar]
- Birnie DH, Holme ER, McKay IC, Hood S, McColl KE, Hillis WS. Association between antibodies to heat shock protein 65 and coronary atherosclerosis. Possible mechanism of action of Helicobacter pylori and other bacterial infections in increasing cardiovascular risk. Eur Heart J 1998; 19(3):387-94. doi: 10.1053/euhj.1997.0618 [Crossref] [ Google Scholar]
- Kanbay M, Gür G, Yücel M, Yilmaz U, Boyacioğlu S. Does eradication of Helicobacter pylori infection help normalize serum lipid and CRP levels?. Dig Dis Sci 2005; 50(7):1228-31. doi: 10.1007/s10620-005-2764-9 [Crossref] [ Google Scholar]
- Blum A, Tamir S, Mualem K, Ben-Shushan RS, Keinan-Boker L, Paritsky M. Endothelial dysfunction is reversible in Helicobacter pylori-positive subjects. Am J Med 2011; 124(12):1171-4. doi: 10.1016/j.amjmed.2011.08.015 [Crossref] [ Google Scholar]
- Coskun S, Kasirga E, Yilmaz O, Bayindir P, Akil I, Yuksel H. Is Helicobacter pylori related to endothelial dysfunction during childhood?. Pediatr Int 2008; 50(2):150-3. doi: 10.1111/j.1442-200X.2008.02542.x [Crossref] [ Google Scholar]
- Oshima T, Ozono R, Yano Y, Oishi Y, Teragawa H, Higashi Y. Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J Am Coll Cardiol 2005; 45(8):1219-22. doi: 10.1016/j.jacc.2005.01.019 [Crossref] [ Google Scholar]
- Sipponen P, Laxén F, Huotari K, Härkönen M. Prevalence of low vitamin B12 and high homocysteine in serum in an elderly male population: association with atrophic gastritis and Helicobacter pylori infection. Scand J Gastroenterol 2003; 38(12):1209-16. doi: 10.1080/00365520310007224 [Crossref] [ Google Scholar]
- Tamura A, Fujioka T, Nasu M. Relation of Helicobacter pylori infection to plasma vitamin B12, folic acid, and homocysteine levels in patients who underwent diagnostic coronary arteriography. Am J Gastroenterol 2002; 97(4):861-6. doi: 10.1111/j.1572-0241.2002.05601.x [Crossref] [ Google Scholar]
- Santarelli L, Gabrielli M, Cremonini F, Santoliquido A, Candelli M, Nista EC. Atrophic gastritis as a cause of hyperhomocysteinaemia. Aliment Pharmacol Ther 2004; 19(1):107-11. doi: 10.1046/j.1365-2036.2003.01820.x [Crossref] [ Google Scholar]
- Kutluana U, Simsek I, Akarsu M, Kupelioglu A, Karasu S, Altekin E. Is there a possible relation between atrophic gastritis and premature atherosclerosis?. Helicobacter 2005; 10(6):623-9. doi: 10.1111/j.1523-5378.2005.00356.x [Crossref] [ Google Scholar]
- Murray LJ, Bamford KB, O’Reilly DP, McCrum EE, Evans AE. Helicobacter pylori infection: relation with cardiovascular risk factors, ischaemic heart disease, and social class. Br Heart J 1995; 74(5):497-501. doi: 10.1136/hrt.74.5.497 [Crossref] [ Google Scholar]
- Hoffmeister A, Rothenbacher D, Bode G, Persson K, März W, Nauck MA. Current infection with Helicobacter pylori, but not seropositivity to Chlamydia pneumoniae or cytomegalovirus, is associated with an atherogenic, modified lipid profile. Arterioscler Thromb Vasc Biol 2001; 21(3):427-32. doi: 10.1161/01.atv.21.3.427 [Crossref] [ Google Scholar]
- Laurila A, Bloigu A, Näyhä S, Hassi J, Leinonen M, Saikku P. Association of Helicobacter pylori infection with elevated serum lipids. Atherosclerosis 1999; 142(1):207-10. doi: 10.1016/s0021-9150(98)00194-4 [Crossref] [ Google Scholar]
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 2009; 6(6):399-409. doi: 10.1038/nrcardio.2009.55 [Crossref] [ Google Scholar]
- Gillum RF. Infection with Helicobacter pylori, coronary heart disease, cardiovascular risk factors, and systemic inflammation: the Third National Health and Nutrition Examination Survey. J Natl Med Assoc 2004; 96(11):1470-6. [ Google Scholar]
- de Luis DA, Lahera M, Cantón R, Boixeda D, San Román AL, Aller R. Association of Helicobacter pylori infection with cardiovascular and cerebrovascular disease in diabetic patients. Diabetes Care 1998; 21(7):1129-32. doi: 10.2337/diacare.21.7.1129 [Crossref] [ Google Scholar]
- Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter 2011; 16(2):79-88. doi: 10.1111/j.1523-5378.2011.00822.x [Crossref] [ Google Scholar]
- Migneco A, Ojetti V, Specchia L, Franceschi F, Candelli M, Mettimano M. Eradication of Helicobacter pylori infection improves blood pressure values in patients affected by hypertension. Helicobacter 2003; 8(6):585-9. doi: 10.1111/j.1523-5378.2003.00180.x [Crossref] [ Google Scholar]
- Adachi K, Arima N, Takashima T, Miyaoka Y, Yuki M, Ono M. Pulse-wave velocity and cardiovascular risk factors in subjects with Helicobacter pylori infection. J Gastroenterol Hepatol 2003; 18(7):771-7. doi: 10.1046/j.1440-1746.2003.03059.x [Crossref] [ Google Scholar]
- Gabrielli M, Santoliquido A, Cremonini F, Cicconi V, Candelli M, Serricchio M. CagA-positive cytotoxic H. pylori strains as a link between plaque instability and atherosclerotic stroke. Eur Heart J 2004; 25(1):64-8. doi: 10.1016/j.ehj.2003.10.004 [Crossref] [ Google Scholar]
- Keikha M, Eslami M, Yousefi B, Ghasemian A, Karbalaei M. Potential antigen candidates for subunit vaccine development against Helicobacter pylori infection. J Cell Physiol 2019; 234(12):21460-70. doi: 10.1002/jcp.28870 [Crossref] [ Google Scholar]
- Ando T, Ishikawa T, Takagi T, Imamoto E, Kishimoto E, Okajima A. Impact of Helicobacter pylori eradication on circulating adiponectin in humans. Helicobacter 2013; 18(2):158-64. doi: 10.1111/hel.12028 [Crossref] [ Google Scholar]
- De Bastiani R, Gabrielli M, Ubaldi E, Benedetto E, Sanna G, Cottone C. High prevalence of Cag-A positive H. pylori strains in ischemic stroke: a primary care multicenter study. Helicobacter 2008; 13(4):274-7. doi: 10.1111/j.1523-5378.2008.00610.x [Crossref] [ Google Scholar]
- Kowalski M. Kowalski MHelicobacter pylori (Hpylori) infection in coronary artery disease: influence of Hpylori eradication on coronary artery lumen after percutaneous transluminal coronary angioplastyThe detection of Hpylori specific DNA in human coronary atherosclerotic plaque. J Physiol Pharmacol 2001; 52(1 Suppl 1):3-31. [ Google Scholar]
- Huang B, Chen Y, Xie Q, Lin G, Wu Y, Feng Y. CagA-positive Helicobacter pylori strains enhanced coronary atherosclerosis by increasing serum OxLDL and HsCRP in patients with coronary heart disease. Dig Dis Sci 2011; 56(1):109-14. doi: 10.1007/s10620-010-1274-6 [Crossref] [ Google Scholar]
- Whincup P, Danesh J, Walker M, Lennon L, Thomson A, Appleby P. Prospective study of potentially virulent strains of Helicobacter pylori and coronary heart disease in middle-aged men. Circulation 2000; 101(14):1647-52. doi: 10.1161/01.cir.101.14.1647 [Crossref] [ Google Scholar]
- Ibrahim HA, Mohammed MO, Dhahir HA, Mahmood KA, Nuradeen BE. Impact of Helicobacter pylori infection on serum lipid profile and atherosclerosis of carotid artery. Int J Clin Med 2014; 5(15):48661. doi: 10.4236/ijcm.2014.515125 [Crossref] [ Google Scholar]
- Vijayvergiya R, Vadivelu R. Role of Helicobacter pylori infection in pathogenesis of atherosclerosis. World J Cardiol 2015; 7(3):134-43. doi: 10.4330/wjc.v7.i3.134 [Crossref] [ Google Scholar]
- Longo-Mbenza B, Nsenga JN, Mokondjimobe E, Gombet T, Assori IN, Ibara JR. Helicobacter pylori infection is identified as a cardiovascular risk factor in Central Africans. Vasc Health Risk Manag 2012; 6:455-61. doi: 10.2147/vhrm.s28680 [Crossref] [ Google Scholar]
- Park MJ, Choi SH, Kim D, Kang SJ, Chung SJ, Choi SY. Association between Helicobacter pylori seropositivity and the coronary artery calcium score in a screening population. Gut Liver 2011; 5(3):321-7. doi: 10.5009/gnl.2011.5.3.321 [Crossref] [ Google Scholar]
- Jha HC, Prasad J, Mittal A. High immunoglobulin A seropositivity for combined Chlamydia pneumoniae, Helicobacter pylori infection, and high-sensitivity C-reactive protein in coronary artery disease patients in India can serve as atherosclerotic marker. Heart Vessels 2008; 23(6):390-6. doi: 10.1007/s00380-008-1062-9 [Crossref] [ Google Scholar]
- Tamer GS, Tengiz I, Ercan E, Duman C, Alioglu E, Turk UO. Helicobacter pylori seropositivity in patients with acute coronary syndromes. Dig Dis Sci 2009; 54(6):1253-6. doi: 10.1007/s10620-008-0482-9 [Crossref] [ Google Scholar]
- Nikolopoulou A, Tousoulis D, Antoniades C, Petroheilou K, Vasiliadou C, Papageorgiou N. Common community infections and the risk for coronary artery disease and acute myocardial infarction: evidence for chronic over-expression of tumor necrosis factor alpha and vascular cells adhesion molecule-1. Int J Cardiol 2008; 130(2):246-50. doi: 10.1016/j.ijcard.2007.08.052 [Crossref] [ Google Scholar]
- Arias E, Martinetto H, Schultz M, Ameriso S, Rivera S, Lossetti O. Seminested polymerase chain reaction (PCR) for detecting Helicobacter pylori DNA in carotid atheromas. Diagn Mol Pathol 2006; 15(3):174-9. doi: 10.1097/01.pdm.0000213454.45398.2e [Crossref] [ Google Scholar]
- Kilic A, Onguru O, Tugcu H, Kilic S, Guney C, Bilge Y. Detection of cytomegalovirus and Helicobacter pylori DNA in arterial walls with grade III atherosclerosis by PCR. Pol J Microbiol 2006; 55(4):333-7. [ Google Scholar]
- Ciervo A, Mancini F, Sale P, Russo A, Cassone A. Real-time polymerase chain reaction and laser capture microdissection: an efficient combination tool for Chlamydophila pneumoniae DNA quantification and localization of infection in atherosclerotic lesions. Int J Immunopathol Pharmacol 2008; 21(2):421-8. doi: 10.1177/039463200802100222 [Crossref] [ Google Scholar]
- Sulewska A, Modrzejewski W, Kovalchuk O, Kasacka I, Jackowski R, Hirnle T. Attempts to detect Helicobacter pylori in atherosclerotic plaques. Rocz Akad Med Bialymst 2004; 49 Suppl 1:239-41. [ Google Scholar]
- Rahmani Y, Mohammadi S, Babanejad M, Rai A, Zalei B, Shahmohammadi A. Association of Helicobacter pylori with presence of myocardial infarction in iran: a systematic review and meta-analysis. Ethiop J Health Sci 2017; 27(4):433-40. doi: 10.4314/ejhs.v27i4.15 [Crossref] [ Google Scholar]
- Danesh J, Peto R. Risk factors for coronary heart disease and infection with Helicobacter pylori: meta-analysis of 18 studies. BMJ 1998; 316(7138):1130-2. doi: 10.1136/bmj.316.7138.1130 [Crossref] [ Google Scholar]
- Yu XJ, Yang X, Feng L, Wang LL, Dong QJ. Association between Helicobacter pylori infection and angiographically demonstrated coronary artery disease: a meta-analysis. Exp Ther Med 2017; 13(2):787-93. doi: 10.3892/etm.2017.4028 [Crossref] [ Google Scholar]
- Yu M, Zhang Y, Yang Z, Ding J, Xie C, Lu N. Association between Helicobacter pylori infection and stroke: a meta-analysis of prospective observational studies. J Stroke Cerebrovasc Dis 2014; 23(9):2233-9. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.020 [Crossref] [ Google Scholar]
- Mayr M, Kiechl S, Mendall MA, Willeit J, Wick G, Xu Q. Increased risk of atherosclerosis is confined to CagA-positive Helicobacter pylori strains: prospective results from the Bruneck study. Stroke 2003; 34(3):610-5. doi: 10.1161/01.str.0000058481.82639.ef [Crossref] [ Google Scholar]
- Cremonini F, Gabrielli M, Gasbarrini G, Pola P, Gasbarrini A. The relationship between chronic H. pylori infection, CagA seropositivity and stroke: meta-analysis. Atherosclerosis 2004; 173(2):253-9. doi: 10.1016/j.atherosclerosis.2003.12.012 [Crossref] [ Google Scholar]
- Sun J, Rangan P, Bhat SS, Liu L. A meta-analysis of the association between Helicobacter pylori infection and risk of coronary heart disease from published prospective studies. Helicobacter 2016; 21(1):11-23. doi: 10.1111/hel.12234 [Crossref] [ Google Scholar]