Arch Iran Med. 25(3):139-147.
doi: 10.34172/aim.2022.24
Original Article
All-Cause and Cause-Specific Mortality in Middle-Aged Individuals with Positive HBsAg: Findings from a Prospective Cohort Study
Nazgol Motamed-Gorji 1, #  , Sareh Eghtesad 1, #, Maryam Sharafkhah 1, Sahar Masoudi 1, Maryam Darvishian 2, Layli Eslami 1, Abdolsamad Gharavi 1, Masoud Khoshnia 1, Gholamreza Roshandel 3, Amaneh Shayanrad 1, Sanam Hariri 1, Shahin Merat 1, Hossein Poustchi 1, *
, Sareh Eghtesad 1, #, Maryam Sharafkhah 1, Sahar Masoudi 1, Maryam Darvishian 2, Layli Eslami 1, Abdolsamad Gharavi 1, Masoud Khoshnia 1, Gholamreza Roshandel 3, Amaneh Shayanrad 1, Sanam Hariri 1, Shahin Merat 1, Hossein Poustchi 1, *  , Reza Malekzadeh 4, *
, Reza Malekzadeh 4, *
Author information:
1Liver and Pancreatobiliary Diseases Research Center, Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
2Cancer Control Research, BC Cancer Research Center, Vancouver, British Columbia, Canada
3Gastroenterology and Hepatology Research Center, Golestan University of Medical Sciences, Gorgan, Iran
4Digestive Diseases Research Center, Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
*Corresponding Authors: Hossein Poustchi MD, PhD; Liver and Pancreatobiliary Diseases Research Center, Digestive Disease Research Institute, Tehran University of Medical Sciences, N. Kargar St., Tehran 14117, Iran. Tel: + 98-21-82415204; Fax: + 98-21-82415400; Email:
h.poustchi@gmail.com; Reza Malekzadeh, MD; Digestive Diseases Research Institute, Tehran University of Medical Sciences, Shariati Hospital, N. Kargar St., Tehran 14117, Iran. Email:
dr.reza.malekzadeh@gmail.com
#Contributed equally to the work as first authors.
Abstract
Background:
While hepatitis B virus (HBV) is the most prevalent cause of adult liver transplants in Iran, the mortality rates and leading causes of death in HBV patients are not well-understood. This study aimed to investigate all-cause and cause-specific mortality among HBsAg positive individuals in a large Iranian cohort.
Methods:
The Golestan Cohort Study includes 50045 individuals aged 40–75 residing in Iran’s Golestan province, enrolled during 2004–2008. HBsAg test was performed at baseline. For the present study, individuals with hepatitis C coinfection were excluded. All-cause mortality was considered as the primary outcome. The association between HBsAg and different mortality causes was evaluated using Cox proportional hazard models. P value<0.05 was considered significant.
Results:
The current study included 49667 participants. After 11.33 (median) follow-up years, there were 7,686 total deaths, with 635 deaths in the HBsAg positive group. In the multivariate Cox proportional hazard model, HBsAg positive individuals had higher all-cause (adjusted hazard ratio [aHR]=1.15, 95% CI: 1.06–1.24) and liver-related mortality risk (aHR=7.13; 5.19–9.79). Mortality from colorectal and pancreatic cancers was higher among male HBsAg positive participants (aHRs=2.41 and 2.22, respectively). Nevertheless, cardiovascular diseases (CVDs) and extrahepatic malignancies were the leading causes of death among both HBsAg positive and negative individuals, and liver-related deaths contributed to an overall 10% of deaths in HBsAg positive patients.
Conclusion:
HBV is associated with significant mortality risk from different causes in Iranian adults. However, solely focusing on liver outcomes in Iranian HBV patients might result in overlooking non-liver events, especially CVD and extrahepatic cancers.
Keywords: Cohort study, Hepatitis B virus, Iran, Mortality
Copyright and License Information
© 2022 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Motamed-Gorji N, Eghtesad S, Sharafkhah M, Masoudi S, Darvishian M, Eslami L, et al. All-cause and cause-specific mortality in middle-aged individuals with positive hbsag: findings from a prospective cohort study. Arch Iran Med. 2022;25(3):139-147. doi: 10.34172/aim.2022.24
Introduction
Chronic hepatitis B is a major global health issue caused by the hepatitis B virus (HBV). It has been reported that 20%–30% of adult chronic hepatitis B patients develop liver cirrhosis or hepatocellular carcinoma (HCC) during their lifetime.1 Currently, 10 genotypes of HBV have been identified, with genotypes A, B, C and D being the most prevalent types around the world.2 While genotypes B and C are most predominant in Southeast Asia and Oceania, genotypes A and D are widely dispersed around the world, mostly concentrated in North America, Europe, the Mediterranean basin, Africa and the Middle East.3
Given the indolent nature of chronic hepatitis B, HBV-infected individuals usually develop its complications years after acquiring the infection. Hence, attributing mortality to HBV infection and its long-term complications can be challenging.4 On the other hand, while previous studies have suggested higher risks of HCC and liver cirrhosis among HBV-positive individuals, these risks vary tremendously from region to region, and seem to be heavily influenced by different viral (including genotype and viral load) and environmental factors (such as alcohol consumption).5,6 Studies from B and C-predominant areas such as Asian and Oceanic regions report high rates of HBV-related mortality, with the majority of deaths being attributed to liver diseases.7 Less information is available from areas of A and D genotype predominance.
Iran is a country located in the Middle East, with HBV strains purely consisting of the D genotype (D1 subgenotype).8 HBV has been listed as the leading cause of liver transplantation in Iranian adults.9 In a recently conducted meta-analysis, the prevalence of HBsAg (hepatitis B surface antigen) seropositivity in Iranian studies was estimated at 5%, 2.5% and 1.3% in studies from 2004–2006, 2007–2009 and 2010-2013, respectively.10 This meta-analysis included studies from different provinces of Iran, and reported the highest prevalence of HBV in the Golestan province (8.86% and 5.1% in studies from 2006 and 2009, respectively).11,12 Furthermore, the Golestan province is a region located near the North-Eastern international border of Iran, which could contribute to higher exposure from other parts of the world.10,12
Longitudinal studies can have a great impact on better understanding the course of the disease and mortality attributed to HBV infection. Launched in 2004, the Golestan Cohort Study (GCS) is a large population-based cohort in the Golestan province, including about 50 000 Golestani residents who have been followed up annually.13 GCS participants were screened for HBsAg during the cohort enrollment and the positive cases were afterwards entered into a sub-cohort.14 Data from this cohort provides a unique opportunity to explore the mortality associated with genotype D HBV in a sample of Iranian adults. Therefore, the present study aimed to: (1) estimate mortality rates (MRs) of Iranian HBsAg positive individuals participating in GCS, and (2) investigate liver- and non-liver-related mortalities among Iranian HBsAg positive individuals, and compare them with studies from other parts of the world.
Materials and Methods
Golestan Cohort Study
This study used data from GCS, a large population-based cohort conducted in the Golestan province, northeastern Iran. Between the years 2004 and 2008, GCS enrolled 50 045 adults aged 40–75 years, residing throughout Golestan. The enrollment phase of this cohort collected the following data: demographics, nutrition, past medical history, current and past medication use and personal habits including smoking and alcohol consumption. Blood pressure and anthropometric measurements were also made. In addition, laboratory measurements of complete blood count (CBC), creatinine, HBsAg and HCV antibody (HCV Ab) were obtained. Details of GCS were previously published.13 Overall, 3505 HBsAg positive individuals were identified at the GCS enrollment, 95% of whom were not aware of their seropositivity and had not received antiviral treatment prior to the testing. These individuals were afterwards recruited in order to perform complimentary tests. One-third of the HBsAg positive individuals were randomly selected to undergo HBV DNA PCR (polymerase chain reaction) analysis.
Data Collection
GCS questionnaires were completed by trained interviewers, while anthropometry, blood pressure measurements and blood samplings were performed by trained health-care professionals in standard environments. Height and weight were measured with light clothing. Body mass index (BMI) was calculated by dividing weight (kg) by heightsquared (m2). Blood pressure was taken twice on both arms, with a 10-minute interval between the first and the second measurements. All blood samples were taken after at least 12 hours of fasting, and tested for HBsAg using the highly sensitive commercially available Enzygnost® HBsAg 6.0 kit (Siemens Healthcare Diagnostics Products, Marburg, Germany with sensitivity: 100% and specificity: 99.89%) according to the manufacturer’s guidelines.14
HBV DNA was extracted from the serum using the QIAamp DNA Blood Mini Kit (QIAGEN, Valencia, CA, USA) and measured in the Light-Cycler (Roche Diagnostics, Mannheim, Germany) by the artus RealArtTMHBV LC PCR (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions.15
Assessment of Mortality
After enrollment, all GCS participants are contacted annually and their health status is evaluated. In the occurrence of death, a verbal autopsy is completed as follows: all pertinent inpatient and outpatient medical records are collected for review. Afterwards, two independent internists verify the cause of death according to the International Classification of Diseases-10th version (ICD-10) codes. Cases of discrepancy are transferred to a senior internist to determine the cause of death. Details of the GCS follow-up were previously published.16
For the current study, follow-up data up until July 1, 2019 were used and we considered all-cause mortality as our primary outcome. We also investigated different cause-specific deaths. The list of all death-causes (according to ICD-10 codes) included in this study are presented in Supplementary file 1 (Table S1).
Definition of Covariates
All covariates were determined according to the enrollment data. Area of residence was dichotomized as “urban” and “rural”. Participants were also classified, according to their education, into “illiterate” (not having the ability to read and write) and “literate” (others). Ethnicity was categorized as “Turkmen” and “others”, while marital status was dichotomized into “ever married” and “never married”. Socioeconomic status (SES) was classified into “low, “average” and “high” according to the tertiles of a wealth score, previously computed using variables pertaining to the ownership of certain assets.17 Physical activity was categorized into “low”, “moderate” and “vigorous” based on the metabolic equivalent of task (MET, in minutes per week) tertiles.18 Participants were defined as tobacco “users” in the case of having used cigarettes, Naas (a kind of chewable tobacco substance) or hookah at least once a week during a period of 6 months or more, and “others” if otherwise.18 For opium, individuals were also dichotomized into “user” (individuals who had a positive history of weekly consumption of opium for at least six consecutive months) and “others” (otherwise). In the case of alcohol, people were considered “user” if they consumed alcoholic drinks at least once a month for at least 6 months.18
Diabetes was defined as a self-report of diabetes diagnosis, or using anti-diabetic medications. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were determined using the mean of systolic and diastolic pressures of both arms at the second measurement. Participants were assumed hypertensive if they had a mean SBP ≥ 140 mm Hg or mean DBP ≥ 90 mm Hg, or if they took antihypertensive agents.
Cases with positive HBsAg at baseline were defined as the HBV-positive group, and all the individuals with negative HBsAg were considered as HBV-negative. Individuals with positive HCV Ab (378 individuals) were excluded from the current study.
Statistical Analysis
Descriptive analyses were used for reporting baseline characteristics of the population. Values were demonstrated as mean (± standard deviation) for normal quantitative variables, median (interquartile range) for skewed quantitative variables, and as number (and percentage) for qualitative variables. We used the Kolmogorov-Smirnov test for assessing normality of baseline variables. As all the compared variables satisfied the normality assumption, baseline characteristics of quantitative variables were compared between HBV-negative and positive groups using t-test. As the expected values in all of the cells of the contingency tables were above 5%, categorical variables were compared using chi-square test.
All-cause (overall) and cause-specific MRs were calculated per 100 000 person-years (PY) during the follow-up period. Furthermore, age-standardized mortality rates (ASMRs) were computed via direct standardization method, using a standard population.19 The World Population Standard (2000–2025) reported by the WHO was used as the standard population. The WHO Population Standard is especially defined to reflect the expected average age structure of the world’s population during 2000–2025.20
Cause-specific mortalities were first categorized into liver- and non-liver-related mortalities, then by cancer-related and non-cancer-related deaths, and finally into different death causes. The association between HBV and mortality were evaluated using Cox proportional hazard regression models. Time to event was calculated as the interval from the baseline interview (which included the blood sampling and HBsAg test) to death, or to the last follow-up date. All analyses were examined for the proportionality assumption using Schoenfeld residuals, and no violation was observed. Associations were adjusted for relevant confounders including age, sex, BMI, physical activity, ethnicity, wealth score, marital status, residential area, alcohol, tobacco and opium consumption. To obtain the contribution of positive HBsAg to overall and cause-specific mortality, the population attributable fraction (PAF) was calculated using the PUNAF module of the Stata software. Interaction analysis was performed using likelihood ratio test, and p for interaction was considered significant at P value < 0.1. Data analyses were performed with Stata Statistical Software, Release 12 (College Station, TX: Stata Corp LLC). Figure plots were depicted using R 3.6.0 for Windows and Microsoft Word 2013. Statistical significance was considered at P value < 0.05.
Results
Of the 50 045 GCS participants, 3,505 were HBV-positive. After excluding HCV Ab-positive individuals, 49 667 total participants with a median follow-up period of 11.33 years (interquartile range = 1.66) remained, 3477 of whom were HBV-positive.
Table 1 shows the baseline characteristics of GCS participants categorized by their HBV status. The total population consisted of 42.31% males, and 79.96% rural residents. The age at baseline was similar in HBV-negative and positive groups. Compared to HBV-negative individuals, the HBV-positive group included a significantly higher percentage of males, rural residents, and individuals of Turkmen ethnicity. Low physical activity, alcohol consumption, tobacco and opium use were also higher in the HBV-positive group, while having a lower prevalence of obesity. Overall, 1148 individuals underwent the viral load test, 66.29% of them had an HBV DNA PCR of less than 2000 IU/mL.
Table 1.
Baseline Characteristics of Golestan Cohort Study Participants Categorized by HBV Status (n = 49 667)
|
Characteristics
|
HBV –
(n=46190)
|
HBV+
(n=3477)
|
P
Value
|
| Age (year), mean (SD) |
52.06 (8.93) |
52.09 (8.61) |
0.83 |
| Male, n (%) |
19 106 (41.36) |
1910 (54.93) |
< 0.001 |
| Rural residence, n (%) |
36 739 (79.54) |
2973 (85.5) |
< 0.001 |
| Illiteracy, n (%) |
32 441 (70.23) |
2436 (70.06) |
0.829 |
| Turkmen Ethnicity, n (%) |
34 200 (74.04) |
2783 (80.04) |
< 0.001 |
| Ever married, n (%) |
40 496 (87.67) |
3128 (89.96) |
< 0.001 |
| Socioeconomic status, n (%) |
Low |
17 676 (38.27) |
1377 (39.6) |
< 0.001 |
| Average |
12 380 (26.80) |
1027 (29.54) |
| High |
16 134 (34.93) |
1073 (30.86) |
| BMI, n (%) |
< 25 |
18 587 (40.24) |
1547(44.49) |
< 0.001 |
| 25-29.9 |
15 745 (34.09) |
1125 (32.35) |
| ≥ 30 |
11 850 (25.65) |
811 (23.32) |
| Physical activity, n (%) |
Low |
15 990 (34.62) |
1459 (41.96) |
< 0.001 |
| Moderate |
14 688 (31.8) |
1016 (29.22) |
| Vigorous |
15 403 (33.35) |
996 (28.64) |
| Alcohol user, n (%) |
1532 (3.32) |
156 (4.49) |
0.001 |
| Tobacco user, n (%) |
9728 (21.06) |
952(27.38) |
< 0.001 |
| Opium user, n (%) |
7590 (16.43) |
750 (21.57) |
< 0.001 |
| Diabetes, n (%) |
3303 (7.15) |
230 (6.61) |
0.236 |
| Hypertension, n (%) |
19 762 (41.78) |
1427 (41.04) |
0.096 |
| HBV DNA PCR* (IU/mL), median (IQR) |
— |
489.5 (5092.5) |
— |
| HBV DNA PCR*, n (%) |
Undetectable |
— |
314 (27.35) |
— |
| Detectable |
< 2000 |
447 (38.94) |
| ≥ 2000 |
387 (33.7) |
HBV: Hepatitis B virus; BMI, body mass index (kg/m2); IQR, interquartile range.
*n = 2329.
The ten leading causes of death in HBV-negative and positive individuals are presented in Figure 1. The four leading causes of death were similar in HBV-positive and negative groups, namely cardiovascular disease (CVD), extrahepatic cancers, respiratory diseases and injuries. CVD deaths were the most common cause in both groups (36.69% and 45.69% of deaths in HBV-positive and negative groups, respectively). Non-cancer and cancer liver-related deaths were the fifth and sixth causes of death among HBV-positive individuals, respectively. Altogether, liver-related causes comprised 10% of deaths in the HBV-positive group, while accounting for 2% of deaths in HBV-negative individuals.
All-cause and cause-specific MRs of HBV-positive and negative participants are presented in Table 2. There were 7686 overall deaths over 567 197 PYs in the total study population, with 7021 confirmed causes of deaths, resulting in an all-cause MR of 1362 per 100 000 PYs, and ASMR of 1945.42 per 100 000 PYs. All-cause ASMR was higher in the HBV-positive group compared to HBV-negative individuals, as were the ASMRs from liver cancer, non-cancer liver diseases and overall non-liver cancers. In the crude model, all-cause mortality was 1.21 times higher in the HBV-positive group compared to the HBV-negative group.
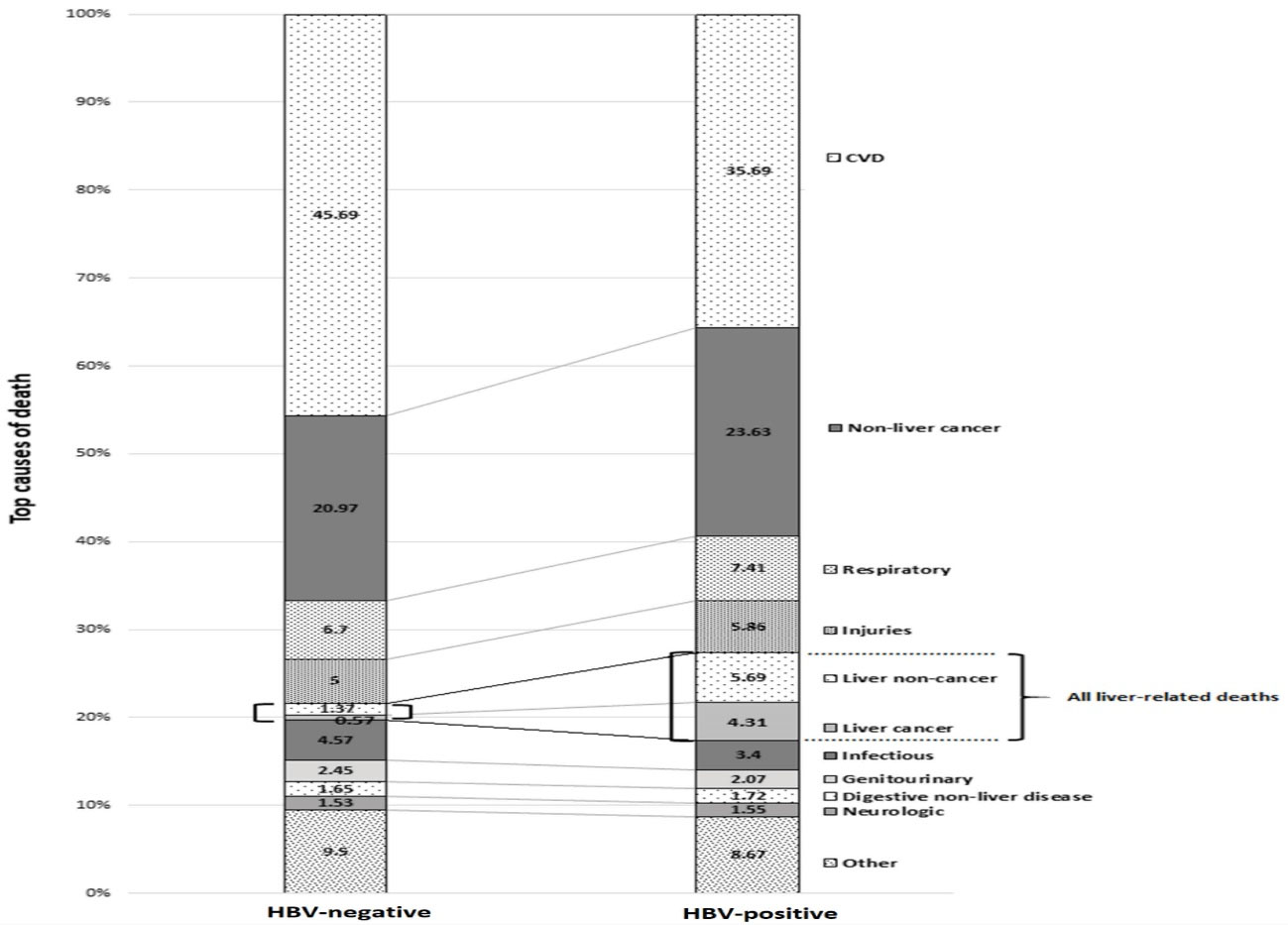
Figure 1.
Top 10 Causes of Death in HBV-Negative and HBV-Positive Participants of GCS during 12 Years of Follow up. HBV, Hepatitis B virus; GCS, Golestan Cohort Study.
.
Top 10 Causes of Death in HBV-Negative and HBV-Positive Participants of GCS during 12 Years of Follow up. HBV, Hepatitis B virus; GCS, Golestan Cohort Study.
Table 2.
All-Cause and Cause-Specific Mortality in Participants of Golestan Cohort Study Categorized by HBV Status
|
Causes of Death
|
HBV-
(n=46190)
|
HBV+
(n=3477)
|
Crude HR(95% CI)
|
|
n
|
MR
|
ASMR
|
n
|
MR
|
ASMR
|
|
All Causes
|
7051 |
1342.3 |
1918.58 |
635 |
1628.84 |
2312.76 |
1.21 (1.13–1.33) |
|
Liver
|
110 |
20.49 |
27.42 |
61 |
157.79 |
195.63 |
7.7 (5.49–10.27) |
|
• Cancer
|
35 |
6.76 |
9.15 |
26 |
68.72 |
88.24 |
10.17 (6.05–16.68) |
|
• Non-caner
|
75 |
14.19 |
17.09 |
37 |
94.17 |
112.36 |
6.64 (4.51–9.92) |
|
Non-liver
|
6305 |
1197.81 |
1721.76 |
527 |
1351.43 |
1957.93 |
1.13 (1.04–1.25) |
|
• Cancers
|
1347 |
257.04 |
347.94 |
140 |
356.31 |
504.49 |
1.39 (1.17–1.68) |
| Digestive organs |
727 |
138.85 |
196.64 |
77 |
195.97 |
280.52 |
1.41 (1.11–1.78) |
| Esophagus |
257 |
48.66 |
71.41 |
26 |
66.17 |
95.45 |
1.36 (0.91–2.03) |
| Stomach |
272 |
52.23 |
75.33 |
27 |
68.72 |
92.32 |
1.32 (0.88–1.95) |
| Colorectal |
75 |
14.09 |
18.43 |
11 |
28 |
38.62 |
1.99 (1.06–3.74) |
| Gall bladder and extrahepatic biliary tract |
34 |
6.58 |
8.43 |
3 |
7.64 |
11.58 |
1.16 (0.36–3.80) |
| Pancreas |
79 |
15.41 |
20.91 |
9 |
22.91 |
38.62 |
1.49 (0.74–2.95) |
| Respiratory and intrathoracic organs |
130 |
24.8 |
33.85 |
11 |
28 |
40.23 |
1.13 (0.61–2.09) |
| Breast and female genitalia |
95 |
18.23 |
17.89 |
7 |
17.82 |
16.51 |
0.98 (0.45–2.10) |
| Stated or presumed to be primary, of lymphoid, hematopoietic and related tissue |
101 |
19.16 |
26.15 |
11 |
28 |
38.92 |
1.46 (0.78–2.71) |
| Other cancers |
294 |
55.99 |
73.4 |
34 |
86.53 |
128.32 |
1.55 (1.08–2.20) |
|
Non-cancer diseases
|
4963 |
941.34 |
1374.39 |
388 |
997.67 |
1455.29 |
1.06 (0.96–1.19) |
| Cardiovascular system (CVD) |
2965 |
560.86 |
812.04 |
211 |
544.64 |
811.26 |
0.97 (0.84–1.12) |
| Digestive system |
110 |
20.86 |
30.71 |
10 |
25.45 |
28.95 |
1.22 (0.6–2.5) |
| Respiratory system |
437 |
82.74 |
132.3 |
47 |
119.62 |
174.78 |
1.45 (1.04–1.94) |
| Genitourinary system |
157 |
29.69 |
44.17 |
13 |
33.09 |
42.53 |
1.11 (0.53–1.89) |
| Nervous system |
102 |
19.16 |
30.74 |
9 |
22.91 |
49.18 |
1.2 (0.56–2.51) |
| Injuries |
321 |
61.06 |
68.63 |
35 |
89.08 |
97.23 |
1.46 (1–2.09) |
| Infectious diseases |
285 |
53.92 |
82.84 |
20 |
50.9 |
75.6 |
0.94 (0.83–1.36) |
| Other non-cancer diseases |
611 |
115.93 |
179.11 |
48 |
124.71 |
189.98 |
1.08 (0.96–1.19) |
HBV, Hepatitis B Virus; CVD, cardiovascular diseases; MR, Mortality rate; ASMR, Age-standardized mortality rate; HR, Hazard ratio.
Table 3 demonstrates the association of HBV with different mortality causes in the adjusted Cox proportional hazard model. HBV positivity was significantly associated with higher all-cause, liver cancer, liver non-cancer and extrahepatic cancer mortality in the full-adjusted model. Among different extrahepatic cancer mortalities, HBV-positive status was only associated with higher colorectal cancer mortality (adjusted hazard ratio [aHR] = 1.93, 1.02-3.66). We did not observe any elevated risk of mortality due to non-Hodgkin lymphoma, gallbladder cancer, lung cancer and breast cancer in HBV-positive patients compared to HBV-negative individuals (data not shown). The total PAF for HBV was 1.1% for overall deaths, 38.58% for liver cancer deaths and 26.16% for liver non-cancer deaths.
Table 3.
HBV Mortality Association with Mortality in Full-Adjusted Cox Proportional Hazard Model in Male, Female and Total Populations
|
Causes of Death
|
Total Population
|
Sex Stratification
|
|
aHR
*
(95% CI)
|
PAF (%)
|
Male aHR (95% CI)
|
Female aHR (95% CI)
|
P
for Interaction
|
|
All Causes
|
1.15 (1.06–1.24) |
1.1 (0.04 to 1.74) |
1.19 (1.08–1.32) |
1.07 (0.94–1.23) |
0.277 |
|
Liver
|
7.13 (5.19–9.79) |
29.41 (21.08 to 36.45) |
9.09 (6.14–13.48) |
4.33 (2.39–7.82) |
0.046 |
|
Cancer
|
9.45 (5.65–15.84) |
38.58 (23.86 to 51.08) |
15.8 (8.18–30.4) |
2.56 (0.75–8.68) |
0.009 |
|
Non-caner
|
6.43 (4.32–9.59) |
26.16 (16.43 to 34.02) |
6.45 (3.87–10.75) |
6.4 (3.37–12.17) |
0.961 |
|
Non-liver
|
1.06 (0.97–1.16) |
0.4 (–0.25 to 1.12) |
1.1 (0.98–1.22) |
0.99 (0.85–1.16) |
0.357 |
|
Cancers
|
1.27 (1.07–1.52) |
2.04 (0.41 to 3.64) |
1.32 (1.07–1.63) |
1.18 (0.86–1.6) |
0.544 |
| Digestive organs |
1.26 (1–1.6) |
1.99 (–0.24 to 4.19) |
1.36 (1.04–1.79) |
1.00 (0.62–1.61) |
0.248 |
| Esophagus |
1.22 (0.82–1.83) |
1.7 (–2.07 to 5.37) |
1.20 (0.73–1.99) |
1.27 (0.65–2.52) |
0.890 |
| Stomach |
1.11 (0.75–1.65) |
0.91 (–2.66 to 4.37) |
1.12 (0.72–1.73) |
1.02 (0.41–2.52) |
0.862 |
| Colorectal |
1.93 (1.02–3.66) |
6.31 (–1.78 to 13.76) |
2.41 (1.17–4.99) |
0.97 (0.23–4.06) |
0.248 |
| Gall bladder and extrahepatic biliary tract |
1.14 (0.35–3.73) |
0.97 (–8.74 to 9.82) |
1.39(0.31–6.11) |
0.84 (0.11–6.30) |
0.718 |
| Pancreas |
1.43 (0.72–2.86) |
3.07 (–3.94 to 9.61) |
2.22 (1.03–4.76) |
0.39 (0.05–2.88) |
0.112 |
| Respiratory and intrathoracic organs |
0.94 (0.51–1.75) |
-0.4 (–5.48 to 4.3) |
1.00 (0.52–1.92) |
0.56 (0.07–4.09) |
0.617 |
| Breast and female genitalia |
1.19 (0.55–2.57) |
1.1 (–4.18 to 6.12) |
— |
1.19 (0.55–2.57) |
— |
| Stated or presumed to be primary, of lymphoid, hematopoietic and related tissue |
1.47 (0.79–5.75) |
3.19 (–3.02 to 9.03) |
1.25 (0.53–2.92) |
1.77 (0.71–4.47) |
0.587 |
| Other cancers |
1.44 (1.01–2.06) |
3.2 (–0.44 to 6.72) |
1.43 (0.91–2.23) |
1.49 (0.82–2.69) |
0.908 |
|
Non-cancer diseases
|
1 (0.9–1.11) |
0 (–0.75 to 0.75) |
1.03 (0.9–1.17) |
0.95 (0.8–1.13) |
0.543 |
| Cardiovascular system (CVD) |
0.93 (0.81–1.06) |
-0.53 (–1.49 to 0.41) |
0.91 (0.76–1.09) |
0.96 (0.77–1.19) |
0.663 |
| Digestive system |
1.14 (0.6–2.19) |
1.04 (–4.4 to 6.21) |
0.92 (0.37–2.3) |
1.53 (0.61–3.84) |
0.454 |
| Respiratory system |
1.3 (0.96–1.76) |
2.23 (–0.63 to 5.02) |
1.31 (0.91–1.9) |
1.34 (0.79–2.27) |
0.868 |
| Genitourinary system |
1.12 (0.63–1.98) |
0.83 (–3.56 to 5.03) |
1.36 (0.65–2.85) |
0.88 (0.36–2.17) |
0.518 |
| Nervous system |
1.16 (0.58–2.29) |
1.13 (–4.68 to 6.62) |
1.3 (0.56–3.05) |
0.96 (0.3–3.1) |
0.673 |
| Injuries |
1.23 (0.86–1.75) |
1.8 (–1.63 to 5.11) |
1.26 (0.84–1.88) |
0.11 (0.52–2.4) |
0.754 |
| Infectious diseases |
0.9 (0.57–1.42) |
-0.74 (–3.81 to 2.24) |
1.07 (0.63–1.82) |
0.62 (0.25–1.5) |
0.307 |
| Other non-cancer diseases |
1.02 (0.76–1.36) |
0.12 (–2.04 to 2.23) |
1.16 (0.82–1.64) |
0.78 (0.46–1.34) |
0.22 |
HBV, Hepatitis B Virus; PAF, Population attributable fraction; CVD, cardiovascular diseases.
*Hazard ratio adjusted for age, sex, BMI, physical activity, ethnicity, wealth score, marital status, residential area, alcohol, tobacco and opium consumption in total population.
Figure 2 illustrates the interaction between HBV and other risk factors in liver and non-liver mortality. Among different potential variables including age, sex, BMI, diabetes, hypertension, alcohol, tobacco and opium use, only sex showed a significant interaction with HBV in liver mortality. In order to further examine the relationship between HBV mortality and sex, we stratified all mortality causes according to the participants’ sex (Table 3). It became clear that the significant interaction between sex and HBV in liver-mortality arose from a positive interaction in liver cancer mortalities (P for interaction = 0.009), in which the risk for men was significantly higher compared to women (aHR = 15.8, 8.18-30.4 in men vs. 2.56, 0.75-8.68 in women).
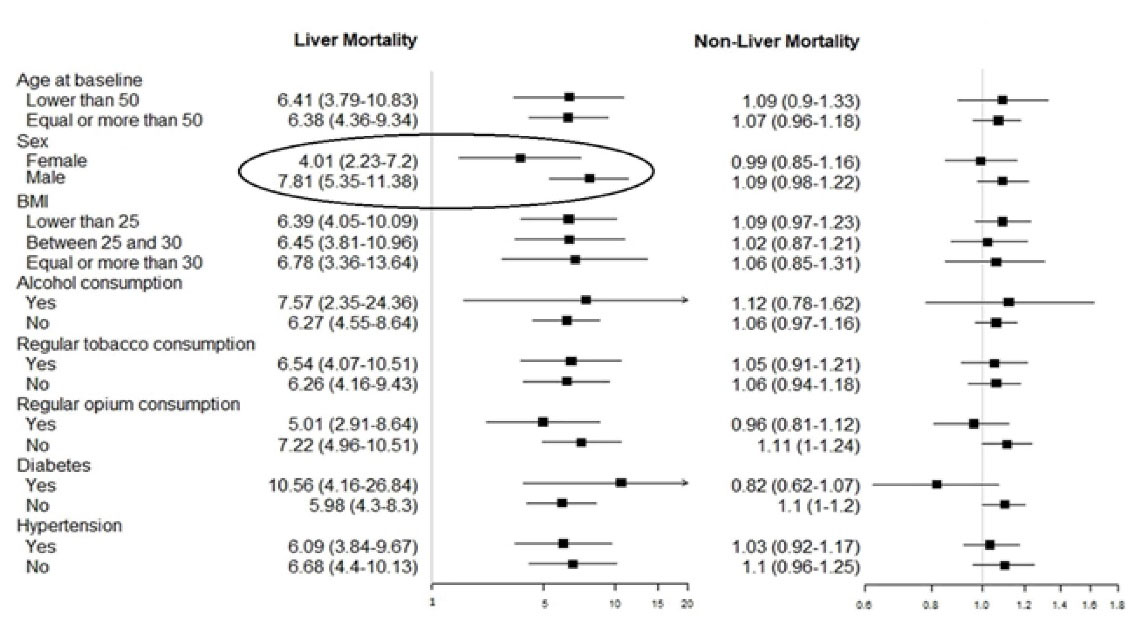
Figure 2.
Forest-Plot Graph of Interaction Analysis. The circled variable (sex) had a significant interaction (P for interaction < 0.1, not stated in the graph) with liver mortality.
.
Forest-Plot Graph of Interaction Analysis. The circled variable (sex) had a significant interaction (P for interaction < 0.1, not stated in the graph) with liver mortality.
The association between HBV and colorectal cancer mortality was also only significant among men and not women; although the interaction of sex was not significant for this category (P for interaction = 0.248). Furthermore, it was demonstrated that HBV seropositivity was significantly associated with higher risk of pancreatic cancer in men but not women; the interaction with sex, however, was again not significant (P for interaction = 0.112).
Discussion
HBV is an ancient virus, tracing back to at least 7000 years ago, with a diverse genetic distribution across different geographical areas.21 Strains of HBV identified in Iran purely consist of genotype D,8 which is mostly predominant in the Middle East, the Mediterranean basin, Europe and Africa.3 In the present study, HBV was associated with 15% increased risk of all-cause mortality, attributed to higher risk of mortality from liver-related causes and certain extrahepatic malignancies.
Despite causing a small percentage of overall deaths in our HBV-positive patients (10%), the relative risk of liver-mortality was significantly associated with HBsAg status. While the male HBV-positive group had increased risk of mortality from cancer and non-cancer liver diseases, the female counterparts showed higher mortality only from non-cancer liver diseases. Similar to our results, Crook et al reported higher risk of liver cancer mortality among male HBV-positive individuals, while female counterparts demonstrated no association with liver cancer death.22 The interaction between sex and liver-cancer mortality in HBV-positive patients was specifically addressed in a 2009 study, which suggested that the male gender and HBsAg seropositivity have a combined synergistic effect on mortality from liver cancer, which is independent of alcohol consumption, tobacco smoking and occupational risk factors.23 This gender disparity has been attributed to different sex hormones.24
In addition to liver mortality, the risks of mortality from colorectal and pancreatic cancers were also higher in our male HBV-positive individuals. The mechanisms underlying the association of HBV and extrahepatic neoplasms are not fully elucidated; however, HBV X protein has been suggested as a possible activator of several oncogenic signaling pathways, including P53 tumor-suppressor and Adenomatous-Polyposis-Coli regulation,25 as well as being involved in the transformation of colorectal adenoma to adenocarcinoma.26 A study in China also showed the risk of colorectal cancer mortality among HBV-positive individuals to be 1.36-fold.27 Similarly, the association between HBV infection and pancreatic adenocarcinoma has been suggested by previous studies.28,29 HBV DNA has been detected in the pancreatic tissues of HBV-positive patients, leading to speculations that HBV is capable of replication within pancreatic cells.30 Overall, these studies support our findings of a possible association between HBV-seropositivity and risk of developing certain cancers; however, due to the small number of both colorectal (n = 11) and pancreatic cancer (n = 9) deaths in our HBV-positive patients, these findings should be interpreted with caution. Future studies should target the association between HBV and development of extrahepatic malignancies.
The overall prognosis of Iranian HBV-positive individuals seems to be better, compared to their counterparts from many Asia-Pacific (China, Palau),7,31 European (Sweden, UK, France)22,32,33 and North American (USA, Canada)34,35 countries, as our liver-related mortality risks are modest in comparison to those reported in studies from the aforementioned areas. This was reflected in GCS mortality findings, in which liver-related deaths were not among the ten leading causes of death in the total population.18 It could be concluded that even the increased risk in liver mortality among HBV-positive individuals does not succeed in placing liver-related causes among the leading causes of death in the total population.
Previous studies suggest that different factors can predict the risk of HCC and cirrhosis development in HBV-positive patients; these factors fall into viral (e.g. viral load, genotype) and environmental (e.g. alcohol consumption, aflatoxin exposure) categories.5,6 The lower liver-related mortality observed in HBV-positive individuals of our study may be justified with regards to these factors.
Few studies have compared HCC incidence across the B, C and D genotypes, suggesting higher carcinogenicity for the C genotype compared to the other two.36,37 A 2013 meta-analysis also showed that the C genotype increases the risk of HCC development 2.34 times compared to A/or D genotypes.38 Therefore, possessing genotype D (the only genotype seen in Iran) could be regarded as a protective factor against the risk of liver mortality, compared to genotype C.
Secondly, different mutations have emerged in the HBV genome due to viral reverse-transcriptase errors.39 It has been shown that the presence of Basal Core Promoter (BCP) double-mutation T1762/A1764 is associated with the development of HCC and cirrhosis,40 and this mutation is observed less often in genotype D, in comparison to C and A.41,42 This could, again, justify why liver-related deaths were not among the top causes of death in our HBV population. In addition, the majority of individuals with available PCR had undetectable or low viral loads. High viral load has been reported as another influencing factor in predicting the development of HCC or cirrhosis.5,43
Apart from the role of viral factors, the incidence and mortality from advanced liver diseases are overall lower in the general population of Iran compared to the average global status.44 This phenomenon could be in part attributed to the low prevalence of alcohol consumption (an important risk factor of liver diseases) in Iran due to religious practices and legal issues.44,45 Alcohol consumption was not among the leading ten level-three risk factors defined by GBD in terms of disability-adjusted life-years in 2015 Iran.46 This was also reflected in a previously published finding that more than 95% of GCS participants had never consumed alcohol regularly.13 Therefore, this could also justify the lower prevalence of liver-related mortality among Golestani HBV-positive individuals compared to studies from areas with higher levels of alcohol consumption. Lower exposure to aflatoxins could be another environmental factor leading to better prognosis of liver outcomes in Iran compared to regions with high levels of exposure, such as South-East Asia and sub-Saharan Africa.47
The present study has some limitations. HBsAg was tested only once at the GCS baseline, and there is the possibility that some positive HBsAg individuals had acute HBV infection. However, having one positive HBsAg strongly indicates chronic state in populations with a predominantly vertical mode of transmission; therefore, in our belief, the effect of acute cases could be disregarded. Performing HBV genotyping, measuring Hepatitis B e-antigen, hepatitis D antibody and human immunodeficiency virus antibody were not possible in our cohort. Also, due to budget limitations, HBV viral load was only performed for one-third of our sample; however, those individuals were randomly selected. Therefore, the available viral loads can be representative of the total population. Furthermore, because of the small number of some of the events (deaths), related HR estimates had wide confidence intervals, which could suggest sparse-data bias.
Our study benefits from several strengths. Our sample was selected from a large population-based cohort study, consisting of both HBV-positive and HBV-negative individuals. Therefore, in contrast to previous studies comparing an HBV-positive sample with the general population, our findings could be presumed stronger. Some studies used the data of the national death registry for their death ascertainments, whereas in our study, death causes were reviewed by at least two different reviewers, using ICD-10 codes. Furthermore, we calculated ASMRs based on the WHO standard population, allowing for a more accurate comparison of HBV morality with other studies. Ultimately, as our sample excluded patients with positive HCV Ab, our findings on the association of HBV and mortality are without the confounding effect of hepatitis C coinfection.
In conclusion, the risk of all-cause mortality was 15% higher among middle-aged Golestani HBV-positive individuals, which was attributed to higher liver-related mortality, as well as colorectal and pancreatic cancer mortality. Despite the higher relative risk of liver deaths in HBV-positive individuals, the majority of deaths in this population occurred due to non-liver causes. Therefore, the most important finding of the current investigation is that focusing on liver outcomes in Iranian HBV-positive individuals should not distract clinicians from close monitoring of the patients for non-liver events, especially CVDs and extrahepatic cancers. Furthermore, we speculate that there may be unknown factors in the population of Iranian HBV patients which are protective against adverse liver outcomes. This could range from the role of specific mutations in the genome of the virus, to the protective effect of some unknown environmental factor.
Supplementary Materials
Supplementary file 1 contains Table S1.
(pdf)
Acknowledgements
Authors would like to thank the personnel of Atrak clinic in Gonbad city for their role in collection of the data. The datasets used for this study are available from the corresponding author on reasonable request. Research reported in this publication was supported by Elite Researcher Grant Committee under award number 977127 from the National Institute for Medical Research Development (NIMAD), Tehran, Iran.
Authors’ Contribution
NM steered the project. MS and SaM analyzed and interpreted the data. NM and SH contributed to the scientific writing of the paper. SE contributed to the scientific writing as well as English editing of the manuscript. LE, AG, MK, GR and AS contributed to data collection. MD and ShM provided expert comments, while HP and RM supervised the whole project. All authors read and approved the final manuscript.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interest.
Ethical Statement
Written informed consent was obtained from all GCS participants at baseline.
13
The present study was ethically approved by the Institutional Review Board (IRB) of the National Institute for Medical Research Development (NIMAD) (Ethical code: IR.NIMAD.REC.1397.409).
Funding
The scientific writing and English editing of this paper by S.E (PhD candidate of Tehran University of Medical Sciences) was funded by the NIMAD [grant’s number: 977127].
References
- Global Hepatitis Report. 2017. Available from https://www.who.int/publications/i/item/global-hepatitis-report-2017. Accessed April 2019.
- Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: recent advances. J Gastroenterol Hepatol 2011; 26 Suppl 1:123-30. doi: 10.1111/j.1440-1746.2010.06541.x [Crossref] [ Google Scholar]
- Norder H, Couroucé AM, Coursaget P, Echevarria JM, Lee SD, Mushahwar IK. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology 2004; 47(6):289-309. doi: 10.1159/000080872 [Crossref] [ Google Scholar]
- Wiktor SZ, Hutin YJ. The global burden of viral hepatitis: better estimates to guide hepatitis elimination efforts. Lancet 2016; 388(10049):1030-1. doi: 10.1016/s0140-6736(16)31018-2 [Crossref] [ Google Scholar]
- Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008; 48(2):335-52. doi: 10.1016/j.jhep.2007.11.011 [Crossref] [ Google Scholar]
- Kao JH. Molecular epidemiology of hepatitis B virus. Korean J Intern Med 2011; 26(3):255-61. doi: 10.3904/kjim.2011.26.3.255 [Crossref] [ Google Scholar]
- Evans AA, Chen G, Ross EA, Shen FM, Lin WY, London WT. Eight-year follow-up of the 90,000-person Haimen City cohort: I. Hepatocellular carcinoma mortality, risk factors, and gender differences. Cancer Epidemiol Biomarkers Prev 2002; 11(4):369-76. [ Google Scholar]
- Ozaras R, Inanc Balkan I, Yemisen M, Tabak F. Epidemiology of HBV subgenotypes D. Clin Res Hepatol Gastroenterol 2015; 39(1):28-37. doi: 10.1016/j.clinre.2014.06.005 [Crossref] [ Google Scholar]
- Malek-Hosseini SA, Jafarian A, Nikeghbalian S, Poustchi H, Bagheri Lankarani K, Nasiri Toosi M. Liver transplantation status in Iran: a multi-center report on the main transplant indicators and survival rates. Arch Iran Med 2018; 21(7):275-82. [ Google Scholar]
- Mohammadi Z, Keshtkar A, Eghtesad S, Jeddian A, Pourfatholah AA, Maghsudlu M. Epidemiological profile of hepatitis B virus infection in Iran in the past 25 years; a systematic review and meta-analysis of general population studies. Middle East J Dig Dis 2016; 8(1):5-18. doi: 10.15171/mejdd.2016.01 [Crossref] [ Google Scholar]
- Abdolahi N, Keshtkar A, Semnani S, Roshandel G, Beshrat S, Joshaghani H. HBV seroprevalence among Golestan adults. Iran J Epidemiol 2006; 2(3):35-40. [ Google Scholar]
- Merat S, Rezvan H, Nouraie M, Jamali A, Assari S, Abolghasemi H. The prevalence of hepatitis B surface antigen and anti-hepatitis B core antibody in Iran: a population-based study. Arch Iran Med 2009; 12(3):225-31. [ Google Scholar]
- Pourshams A, Khademi H, Fazeltabar Malekshah A, Islami F, Nouraei M, Sadjadi AR. Cohort profile: the Golestan Cohort Study--a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol 2010; 39(1):52-9. doi: 10.1093/ije/dyp161 [Crossref] [ Google Scholar]
- Poustchi H, Katoonizadeh A, Ostovaneh MR, Moossavi S, Sharafkhah M, Esmaili S. Cohort profile: Golestan hepatitis B cohort study- a prospective long term study in northern Iran . Middle East J Dig Dis 2014; 6(4):186-94. [ Google Scholar]
- Besharat S, Poustchi H, Mohamadkhani A, Roshandel G, Freedman ND, Merat S. Central obesity and advanced liver stiffness in hepatitis B: result from Golestan hepatitis B cohort study. Arch Iran Med 2015; 18(9):562-6. [ Google Scholar]
- Khademi H, Etemadi A, Kamangar F, Nouraie M, Shakeri R, Abaie B. Verbal autopsy: reliability and validity estimates for causes of death in the Golestan Cohort Study in Iran. PLoS One 2010; 5(6):e11183. doi: 10.1371/journal.pone.0011183 [Crossref] [ Google Scholar]
- Islami F, Kamangar F, Nasrollahzadeh D, Aghcheli K, Sotoudeh M, Abedi-Ardekani B. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol 2009; 38(4):978-88. doi: 10.1093/ije/dyp195 [Crossref] [ Google Scholar]
- Nalini M, Oranuba E, Poustchi H, Sepanlou SG, Pourshams A, Khoshnia M. Causes of premature death and their associated risk factors in the Golestan Cohort Study, Iran. BMJ Open 2018; 8(7):e021479. doi: 10.1136/bmjopen-2018-021479 [Crossref] [ Google Scholar]
- Naing NN. Easy way to learn standardization: direct and indirect methods. Malays J Med Sci 2000; 7(1):10-5. [ Google Scholar]
- Age Standardization of Rates: A New WHO Standard. 2013. Available from: https://seer.cancer.gov/stdpopulations/world.who.html. Accessed March 2019.
- Kostaki EG, Karamitros T, Stefanou G, Mamais I, Angelis K, Hatzakis A. Unravelling the history of hepatitis B virus genotypes A and D infection using a full-genome phylogenetic and phylogeographic approach. Elife 2018; 7. doi: 10.7554/eLife.36709 [Crossref]
- Crook PD, Jones ME, Hall AJ. Mortality of hepatitis B surface antigen-positive blood donors in England and Wales. Int J Epidemiol 2003; 32(1):118-24. doi: 10.1093/ije/dyg039 [Crossref] [ Google Scholar]
- Wang N, Zheng Y, Yu X, Lin W, Chen Y, Jiang Q. Sex-modified effect of hepatitis B virus infection on mortality from primary liver cancer. Am J Epidemiol 2009; 169(8):990-5. doi: 10.1093/aje/kwn418 [Crossref] [ Google Scholar]
- Yu MW, Yang YC, Yang SY, Cheng SW, Liaw YF, Lin SM. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J Natl Cancer Inst 2001; 93(21):1644-51. doi: 10.1093/jnci/93.21.1644 [Crossref] [ Google Scholar]
- Wu CG, Salvay DM, Forgues M, Valerie K, Farnsworth J, Markin RS. Distinctive gene expression profiles associated with hepatitis B virus x protein. Oncogene 2001; 20(28):3674-82. doi: 10.1038/sj.onc.1204481 [Crossref] [ Google Scholar]
- Hsieh A, Kim HS, Lim SO, Yu DY, Jung G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/β-catenin signaling. Cancer Lett 2011; 300(2):162-72. doi: 10.1016/j.canlet.2010.09.018 [Crossref] [ Google Scholar]
- Kamiza AB, Su FH, Wang WC, Sung FC, Chang SN, Yeh CC. Chronic hepatitis infection is associated with extrahepatic cancer development: a nationwide population-based study in Taiwan. BMC Cancer 2016; 16(1):861. doi: 10.1186/s12885-016-2918-5 [Crossref] [ Google Scholar]
- Ben Q, Li Z, Liu C, Cai Q, Yuan Y, Wang K. Hepatitis B virus status and risk of pancreatic ductal adenocarcinoma: a case-control study from China. Pancreas 2012; 41(3):435-40. doi: 10.1097/MPA.0b013e31822ca176 [Crossref] [ Google Scholar]
- Iloeje UH, Yang HI, Jen CL, Su J, Wang LY, You SL. Risk of pancreatic cancer in chronic hepatitis B virus infection: data from the REVEAL-HBV cohort study. Liver Int 2010; 30(3):423-9. doi: 10.1111/j.1478-3231.2009.02147.x [Crossref] [ Google Scholar]
- Dejean A, Lugassy C, Zafrani S, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in pancreas, kidney and skin of two human carriers of the virus. J Gen Virol 1984; 65(Pt 3):651-5. doi: 10.1099/0022-1317-65-3-651 [Crossref] [ Google Scholar]
- Vogt TM, Goldstein ST, Kuartei S. Endemic hepatitis B virus infection and chronic liver disease mortality in the Republic of Palau, 1990-2002. Trans R Soc Trop Med Hyg 2006; 100(12):1130-4. doi: 10.1016/j.trstmh.2006.01.011 [Crossref] [ Google Scholar]
- Duberg AS, Törner A, Daviðsdóttir L, Aleman S, Blaxhult A, Svensson Å. Cause of death in individuals with chronic HBV and/or HCV infection, a nationwide community-based register study. J Viral Hepat 2008; 15(7):538-50. doi: 10.1111/j.1365-2893.2008.00982.x [Crossref] [ Google Scholar]
- Montuclard C, Hamza S, Rollot F, Evrard P, Faivre J, Hillon P. Causes of death in people with chronic HBV infection: a population-based cohort study. J Hepatol 2015; 62(6):1265-71. doi: 10.1016/j.jhep.2015.01.020 [Crossref] [ Google Scholar]
- Bixler D, Zhong Y, Ly KN, Moorman AC, Spradling PR, Teshale EH. Mortality among patients with chronic hepatitis B infection: the chronic hepatitis cohort study (CHeCS). Clin Infect Dis 2019; 68(6):956-63. doi: 10.1093/cid/ciy598 [Crossref] [ Google Scholar]
- Pohani G, Zou S, Tepper M. Trends of hepatitis B and hepatitis C mortality in Canada, 1979-1997. Can J Public Health 2001; 92(4):250-4. doi: 10.1007/bf03404954 [Crossref] [ Google Scholar]
- Gaglio P, Singh S, Degertekin B, Ishitani M, Hussain M, Perrillo R. Impact of the hepatitis B virus genotype on pre- and post-liver transplantation outcomes. Liver Transpl 2008; 14(10):1420-7. doi: 10.1002/lt.21563 [Crossref] [ Google Scholar]
- Madan K, Batra Y, Sreenivas V, Mizokami M, Tanaka Y, Chalamalasetty SB. HBV genotypes in India: do they influence disease severity?. Hepatol Res 2009; 39(2):157-63. doi: 10.1111/j.1872-034X.2008.00417.x [Crossref] [ Google Scholar]
- Wong GL, Chan HL, Yiu KK, Lai JW, Chan VK, Cheung KK. Meta-analysis: the association of hepatitis B virus genotypes and hepatocellular carcinoma. Aliment Pharmacol Ther 2013; 37(5):517-26. doi: 10.1111/apt.12207 [Crossref] [ Google Scholar]
- Lin CL, Kao JH. Hepatitis B virus genotypes and variants. Cold Spring Harb Perspect Med 2015; 5(5):a021436. doi: 10.1101/cshperspect.a021436 [Crossref] [ Google Scholar]
- Liu CJ, Chen BF, Chen PJ, Lai MY, Huang WL, Kao JH. Role of hepatitis B viral load and basal core promoter mutation in hepatocellular carcinoma in hepatitis B carriers. J Infect Dis 2006; 193(9):1258-65. doi: 10.1086/502978 [Crossref] [ Google Scholar]
- Duong TN, Horiike N, Michitaka K, Yan C, Mizokami M, Tanaka Y. Comparison of genotypes C and D of the hepatitis B virus in Japan: a clinical and molecular biological study. J Med Virol 2004; 72(4):551-7. doi: 10.1002/jmv.20044 [Crossref] [ Google Scholar]
- Tanaka Y, Hasegawa I, Kato T, Orito E, Hirashima N, Acharya SK. A case-control study for differences among hepatitis B virus infections of genotypes A (subtypes Aa and Ae) and D. Hepatology 2004; 40(3):747-55. doi: 10.1002/hep.20365 [Crossref] [ Google Scholar]
- Osawa M, Akuta N, Suzuki F, Fujiyama S, Kawamura Y, Sezaki H. Prognosis and predictors of hepatocellular carcinoma in elderly patients infected with hepatitis B virus. J Med Virol 2017; 89(12):2144-8. doi: 10.1002/jmv.24890 [Crossref] [ Google Scholar]
- Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017; 3(12):1683-91. doi: 10.1001/jamaoncol.2017.3055 [Crossref] [ Google Scholar]
- Bagheri Lankarani KB, Afshari R. Alcohol consumption in Iran. Lancet 2014; 384(9958):1927-8. doi: 10.1016/s0140-6736(14)62279-0 [Crossref] [ Google Scholar]
- Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388(10053):1659-724. doi: 10.1016/s0140-6736(16)31679-8 [Crossref] [ Google Scholar]
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M. Hepatocellular carcinoma. Nat Rev Dis Primers 2016; 2:16018. doi: 10.1038/nrdp.2016.18 [Crossref] [ Google Scholar]