Arch Iran Med. 25(3):148-154.
doi: 10.34172/aim.2022.25
Original Article
Magnesium Sulfate for Prevention of Post-ERCP-Pancreatitis: A Randomized Controlled Trial
Najmeh Aletaha 1  , Hoda Hamid 1, *
, Hoda Hamid 1, *  , Abbas Alipour 2, Pardis Ketabi Moghadam 3
, Abbas Alipour 2, Pardis Ketabi Moghadam 3
Author information:
1Gastroenterology Department, Tehran University of Medical Sciences, Tehran, Iran
2Thalassemia Research Center, Mazandaran University of Medical Sciences, Sari, Iran
3Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti Medical University, Tehran, Iran
*Corresponding Author: Hoda Hamid, MD; Gastroenterology Department, Tehran University of Medical Sciences, Iran. Tel:+98-21-61190; Email:
h-hamid@razi.tums.ac.ir
Abstract
Background:
Acute pancreatitis is one of the most common complications of endoscopic retrograde cholangiopancreatography (ERCP). Studies suggest that intrapancreatic calcium has an important role in activating pancreatic enzymes; in addition, elevated intraductal pressure is required for development of pancreatitis. Magnesium sulfate (MS), as a calcium antagonist and a muscle relaxant of the Oddi sphincter, is suggested to reduce the incidence and severity of post-ERCP-pancreatitis (PEP) in this article.
Methods:
We included 270 patients who referred for ERCP between March 2017 and March 2018. They were enrolled into MS (2 g) and placebo (normal saline) groups, administered 1 hour before and 6 hours after the procedure. The ERCPs were done by fellows of gastroenterology under supervision of expert physicians. The incidence and severity of PEP were followed.
Results:
PEP was seen in 12 (8.9%) patients in the MS group and 17 (12.6%) in the placebo group (P value=0.33). The incidence of PEP in high risk patients group (P value=0.017).
Conclusion:
Although the usage of MS was not able to prevent PEP in all patients enrolled in this study, but it could significantly reduce the incidence of PEP in high risk patients of intervention group in comparison with placebo group. The median length of hospital stay was also significantly lower in new drug group in contrast to placebo.
Keywords: Cholangiopancreatography, Endoscopic Retrograde, Magnesium Sulfate, Pancreatitis, Sphincter of Oddi dysfunction
Copyright and License Information
© 2022 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Aletaha N, Hamid H, Alipour A, Ketabi Moghadam P. Magnesium sulfate for prevention of post-ercp-pancreatitis: a randomized controlled trial. Arch Iran Med. 2022;25(3):148-154. doi: 10.34172/aim.2022.25
Introduction
Post-ERCP-pancreatitis (PEP) is one of the most common and overwhelming complications of endoscopic retrograde cholangiopancreatography (ERCP). The incidence of PEP is estimated at 3–10% in systematic review articles.1-3 Some other articles have reported the overall incidence of PEP to be up to 15%.4,5 There are some known risk factors for PEP such as age under 35 years, female gender, normal serum bilirubin level, Sphincter of Oddi dysfunction (SOD), difficult cannulation and history of previous PEP.3,6 Mechanical, chemical and microbial agents can trigger pancreatic enzymes to be activated, resulting in pancreatitis. Papillary manipulation is the most common cause of increasing intraductal pressure, leading to PEP.7 Some drugs such as somatostatin and gabexate mesylate prevent PEP by relaxing the sphincter of Oddi.8 Activation of pancreatic enzymes is calcium dependent.9 Magnesium ions are calcium antagonists, so they can theoretically inhibit pancreatic enzyme activation.10 The physiologic rationale for the effect of magnesium sulfate (MS) on reduction of the frequency and severity of pancreatitis has been depicted in several previous studies.8 As an instance, there are some studies emphasizing the role of high calcium concentration in pancreatitis. They have also claimed that magnesium supplementation can antagonize the pathological signals of calcium ions by reducing the concentration and fluctuation of intracellular calcium. Having reduced calcium ions, magnesium ions can inhibit the premature activity of elastase and trypsin in pancreatic acini.11 In addition, MS can relax the sphincter of by stimulating cholecystokinin release. So, it can decrease the pancreatic intraductal pressure through this mechanism.12 Moreover, MS has been introduced as a purgative agent for precipitating the intestinal transit of pancreatic enzymes.13 PEP might lead to multi-organ failure and even death. So, finding ways to reduce the Oddi frequency and intensity of PEP is crucial and well worth the study.14,15 There are huge numbers of clinical trials evaluating the effect of non-steroidal anti-inflammatory drugs (NSAIDs)16-19 gabexate mesylate,20 epinephrine,9 glyceryl trinitrate,10 risperidone21 and pancreatic stentingon the reduction of PEP, but the results are controversial and non-reproducible, necessitating more new studies in this field. Among the mentioned strategies, NSAIDs and pancreatic stenting are the most acceptable approach in American and European guidelines.4 To answer the mentioned question, we decided to evaluate the effect of MS on decreasing the frequency and intensity of PEP.
Materials and Methods
The present study is a prospective, randomized and double-blind controlled trial on the efficacy of MS in reduction of PEP frequency and intensity along with the length of hospital stay. The study was designed and performed under the supervision of the Iranian Registry of Clinical Trials [IRCTID: IRCT20180310039017N1 (https://www.irct.ir/trial/30177)], and was approved by the ethics committee of Tehran University of Medical Sciences (TUMS). Patients older than 18 years and candidate for ERCP who referred to the gastroenterology ward of Imam hospital, a teaching referral hospital, in Tehran, Iran between March 2017 and March 2018 were enrolled in the study after accepting and signing a written informed consent. Patients with renal failure and glomerular filtration rate (GFR) < 60, heart block, bradycardia, hypotension, baseline amylase more than three-folds the normal ceiling, abnormal baseline serum magnesium level, myasthenia gravis or other neuromuscular diseases, cirrhosis child C, pregnancy, breast feeding, renal stone (calcium or magnesium phosphate), history of Billroth II and other surgeries leading to difficulty in access to papilla, unsuccessful ERCPs after the procedure and hypersensitivity to magnesium were excluded from the study. Any reaction to MgSO4 infusion such as hypotension or flushing which made healthcare providers aware of the drug (confounding with blinding) was considered as an exclusion criterion, too. Patients’ refusal to participate in the trial was another disqualifying factor excluding them from the study. All patients received intravenous (IV) fluid Ringer lactate (3 cc/kg) during ERCP, 20 cc/kg stat just after the procedure and 8 cc/kg/h for the next 8 hours after the procedure. They all took rectal indomethacin 100 mg immediately after ERCP. Patients in the study were randomly allocated into the placebo and drug groups using block randomization to make equal sample sizes. To randomize patients into treatment and placebo groups, a computer program was designed to perform block randomization by obtaining the random numbers and assigning random numbers to each patient in each group. A block size of 4 was used in this computer program. This program made patients, nurses, physicians, endoscopists and other health care workers related to the treatment program unaware of patients’ allocation to drug and placebo arms. The patients were randomly assigned to undergo an infusion of 2 g MS (5 cc of MgSO4 20%) as drug or 5 cc saline 3% as placebo by micro set over 30 minutes, an hour before and 6 hours after ERCP. Both the drug and placebo were 50 cc of transparent liquid in similar vials. The drug information on the vials was concealed and vials were labeled with codes which were used by the computer program for allocation to each arm. The patients were visited by a physician unaware of study groups, 6 hours and 24 hours after the procedure. The procedures were performed by fellows of gastroenterology of the hospital under supervision of well-experienced attending physicians. The fellows were instructed to deliver the scope to the attending physician after 2 unsuccessful attempts for cannulation. Statistical analyses were performed at the center of TUMS. Codes were broken only at the end of the study. Any complaint of abdominal pain compatible with pancreatitis was evaluated by physical examination, serum level of amylase and lipase. Patients who fulfilled two items of the Atlanta criteria22 were considered to have PEP: abdominal pain compatible with pancreatitis, amylase or lipase more than three-folds the normal ceiling and imaging indicating pancreatitis. Patients with PEP were stratified into three groups of mild, moderate and severe pancreatitis based on the Atlanta criteria. Patients with no organ failure, organ failure lasting for no more than 48 hours and organ failure remaining unchanged without any improvement for more than 48 hours or persistent systemic inflammatory response syndrome (SIRS) were assigned to the groups of mild, moderate and severe pancreatitis, respectively. The major risk factors were SOD, previous history of PEP, difficult or failed cannulation (more than 3 attempts), pancreatic duct (PD) contrast injection, pancreatic sphincterotomy, precut sphincterotomy, biliary sphincterotomy of patients harboring suspicion of SOD and ampullectomy. The minor risk factors were female gender, age < 40, normal serum bilirubin, normal common bile duct (CBD) size, history of recurrent pancreatitis, PD brush cytology, contrast injection and endoscopic papillary large bile duct dilation (EPLBD) of intact sphincter. Patients with at least one major risk factor or two minor risk factors were considered high risk for PEP.19 PD stenting and chronic pancreatitis were considered factors with protective effects on PEP. The sample size was calculated at 135 participants for each group of the controlled trial based on a relevant study,23 taking the probability of PEP occurrence into assumption which was considered as 10 and 2 in the placebo and intervention arms, respectively, and also considering the probability of type I and II errors as 0.05 and 0.2, respectively.23 Normal distribution of data was analyzed by the Shapiro-Wilk test. Qualitative variables for the two groups (MS and control) were presented as mean (standard deviation, SD) and quantitative variables were introduced as numbers (percentages). Comparison between the two study groups in terms of consequences was made using the T and Chi-square tests to determine odds ratio (OR) and relative risk (RR) for PEP with 95% confidence intervals (CIs). Additionally, we used a logistic regression model to estimate the association between the study groups and high risk conditions with PEP progression after adjusting for other variables. Survival analysis was used for calculation of median time and hazard ratio (HR). The Stata software version 13 was used for final statistical analysis of the data.
Results
A total of 300 patients who referred to Imam Khomeini hospital, a teaching referral hospital, in Tehran, Iran for ERCP from March 2017 to March, were initially included in the study. Of these, 24 patients did not have the requirements to enter the study and 6 patients did not accept to participate in the study (Figure 1). Finally, a total of 270 patients (135 in each arm) were eligible for the study and randomly received MS or placebo. The baseline demographics and clinical characteristics of each group of patients and the patients’ characteristics which make them high risk for PEP are presented in Table 1. As could be seen, the mean age of patients in the placebo and treatment groups was 58.08 ± 17 and 56.48 ± 15.78, respectively and the difference was not statistically significant (P value = 0.18). According to Table 1, 51.8% of participants in the placebo group and 56.3% of patients in the drug group were females and there was not any statistically significant gender difference between the groups (95% CI: 0.87-1.35, RR: 1.08, P value = 0.46). Fortunately, no reaction to MS was reported during this study and no patient was excluded because of side effects of the new introduced drug. The number of patients under 40 years was not found to be statistically different in the two groups of placebo and drug according to the findings in Table 1 (95% CI: 0.57–1.59, RR:0.96, P value = 0.87). The Chi-square test was performed to examine the difference between the prevalence of high risk characteristics in the two groups of patients. This test revealed no statistically significant difference between the prevalence of high risk characteristics of the two groups (Table 1, P value > 0.05) except for normal serum bilirubin level which was seen to be significantly higher in the drug group, (56 versus 42; 95%CI: 0.96–1.83, RR: 1.33, P value = 0.079). Baseline demographic data were evaluated in high risk patients of the placebo and intervention groups and are presented in Table 2. As could be seen, there was no significant difference between the mean age of high risk patients of the placebo group (51.91 ± 3.28, M = 51.91 ± 18.3) compared with the drug group (52.83 ± 3.02, M = 52.83 ± 17.82, P value = 0.3). Also, the results reported in high risk patients of the placebo and drug groups indicate that the difference between the mentioned risk factors for PEP, such as the number of females and patients under 40 years, was not statistically significant (Table 2, P value = 0.89 and 0.17, respectively). More elaborately, among high risk patients, 73.3% of patients in the placebo arm and 74.3% of patients in the treatment arm were females (Table 2, 95% C: 0.82–1.24, RR: 1.01, P value = 0.89). Also, 36.6% of the placebo group and 25.6% of the drug group were under 40 years of age (Table 2, 95% CI: 0.42–1.16, RR: 0.7, P value = 0.17). The indications for ERCP are depicted in Table 3. As we can see, all of the indications were therapeutic and choledocholithiasis was the most common indication for ERCP in both groups of participants with or without cholangitis. The ERCPs were performed by fellows of gastroenterology under supervision of expert endoscopists of Imam Khomeini hospital, Tehran, Iran. The PEP prevalence in patients who received MS and placebo was 8.9% (12 patients) and 12.6% (17 patients), respectively as depicted in Table 4 (95%CI: 0.31–1.48, OR: 0.68, P value = 0.33). According to Table 4, the proportion of subjects who had PEP did not differ statistically by drug or placebo (P value = 0.33). PEP severity was mild in all patients (100%) of the MS group and 14 patients (82.4%) of the placebo group. Three patients (17.6%) of controls had moderate PEP. Totally, the difference between PEP severity in the two groups of patients as presented in Table 4 was not statistically significant (P value = 0.19). Neither severe pancreatitis nor death was reported in the two arms. Details of pancreatitis intensity for each arm of patients are presented in Table 4. The incidence of PEP among patients at risk for PEP separately in the MS and placebo groups was evaluated to assess the effect of MS in patients with high risk characteristics of PEP. The final results are also seen in Table 4 which show a significant decrease in PEP prevalence in participants with high risk characteristics for PEP in the MS arm in contrast to the placebo group (10.81% (8 patients out of 74 patients) versus 26.67% (16 patients out of 60 patients), respectively; 95% CI: 1.18–7.61, OR:3, P value = 0.017). Based on the data, MS in high risk patients is more likely to decrease the incidence of PEP in comparison with patients without risk factors for PEP. The statistics for low risk patients are also shown in Table 4. As could be seen, the decrease in PEP among low risk patients of both groups was not significant (95% CI: 0.02–1.77, OR: 0.019, P value = 0.26). Based on binary logistic regression which is presented in Table 5, there was not any statistical significant difference between the two arms of patients receiving placebo or drug in terms of the incidence of PEP after adjusting for factors (95% CI: 0.24–1.25, OR: 0.56, P value = 0.16). However, as we can see in Table 5, high risk characteristics of PEP increased the odds of PEP (95% CI: 2.24–1.25, OR: 6.13, P value = 0.0004). So, there was an interaction effect between taking MS and being high risk for PEP in reduction of PEP incidence (95% CI: 0.13–0.84, OR: 0.33, P value = 0.02). As a result, having high risk characteristics for PEP could be said to be a modifier of the effect of MS on the incidence of PEP.
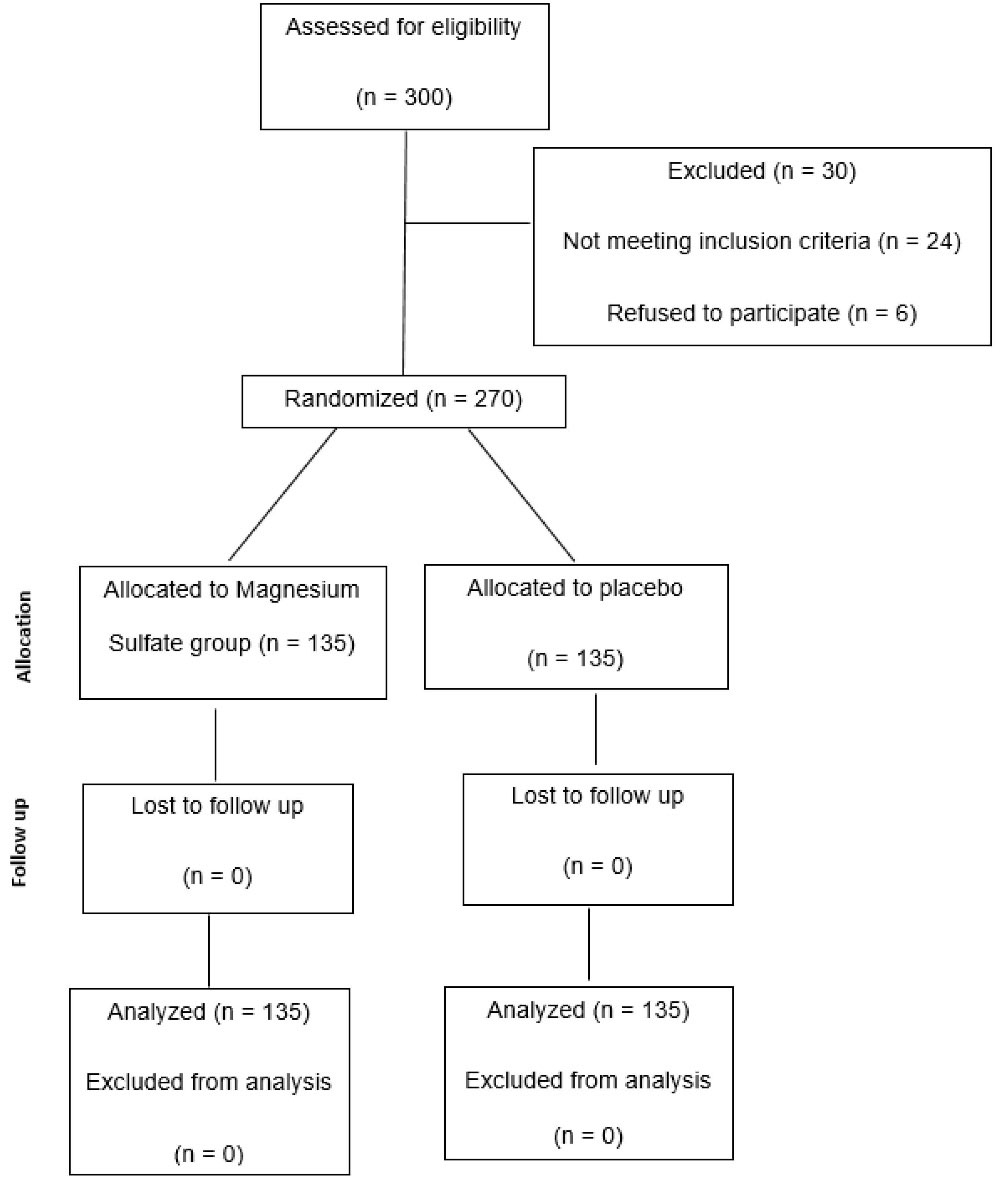
Figure 1.
Flow Diagram of Participants in 2-Group Parallel Randomized Trial.
.
Flow Diagram of Participants in 2-Group Parallel Randomized Trial.
Table 1.
Baseline Demographic Characteristics and Risk-Related Characteristics of Each Group of Participants
|
Participants’ Characteristics
|
Placebo Group
(n=135)
|
MS Group
(n=135)
|
RR
|
P
Value
|
|
Baseline Demographic
|
| Mean age (years) (SD) |
58.08 (± 17.065) |
56.48 (± 15.783) |
— |
0.186 |
| Gender (Female) (n) (%) |
70 (51.85) |
76 (56.3) |
1.085 |
0.464 |
| Number of patients less than 40 years old (%) |
25 (18.5) |
24 (17.8) |
0.96 |
0.874 |
|
Risk-Related Characteristics
|
|
|
Placebo group
(
n
=135)
|
MS group
(
n
=135)
|
95% CI
Confidence Interval
|
RR
|
P
value
|
|
Major risk factors
|
|
|
|
|
|
| SOD, n (%) |
0 (0) |
1 (0.7) |
0.123–72.998 |
3 |
0.499 |
| History of PEP, n (%) |
0 (0) |
1 (0.7) |
0.123–72.998 |
3 |
0.499 |
| PD cannulation more than 3 times, n (%) |
6 (27.3) |
13 (40.6) |
0.848–5.532 |
2.166 |
0.106 |
| Difficult cannulation n (%) |
10 (7.4) |
7 (5.2) |
0.274–1.784 |
0.7 |
0.455 |
| Ampullectomy, n (%) |
0 (0) |
1 (0.7) |
0.123–72998 |
3 |
0.499 |
|
Minor risk factors
|
| Normal CBD size, n (%) |
19 (14.1) |
20 (14.8) |
0.588–1.881 |
1.052 |
0.862 |
| Normal serum bilirubin level, n (%) |
42 (31.1) |
56 (41.5) |
0.967–1.838 |
1.33 |
0.0792 |
| History of recurrent pancreatitis, n (%) |
0 (0) |
1 (0.7) |
0.123–72.998 |
3 |
0.32 |
| PD cannulation, n (%) |
22 (16.2) |
32 (23.7) |
0.893–2.368 |
1.454 |
0.132 |
| EPLBD of intact sphincter, n (%) |
0 (0) |
0 (0) |
— |
— |
— |
| Contrast injection in PD, n (%) |
1 (0.7) |
2 (1.5) |
0.183–21.796 |
2 |
0.569 |
| Age under 40 |
25 (18.5) |
24 (17.8) |
0.578–1.593 |
0.96 |
0.874 |
| Being female |
70 (51.85) |
76 (56.3) |
0.871–1.353 |
1.085 |
0.464 |
|
2. No risk factor
|
|
|
|
|
|
Chronic pancreatitis, n (%)
Negative effect on PEP |
0 (0) |
3 (2.22) |
0.365–134.236 |
7 |
0.196
NA* |
PD stent placement, n (%)
Negative effect on PEP |
8 (5.9) |
7 (5.2) |
0.326–2.345 |
0.875 |
0.79
NA* |
| Total number of high risk patients based on one major risk factor or 2 minor risk factors, n (%) |
60 (44.44) |
74 (54.8) |
0.967–1.572 |
1.273 |
0.090 |
MS, Morphine sulfate; RR, Relative risk; SOD, Sphincter of Oddi dysfunction; PEP, Post-ERCP-pancreatitis; PD, Pancreatic duct; EPLBD, Endoscopic papillary large balloon dilation.
*Not Applied high risk.
Table 2.
Evaluation of Demographic Data Among High Risk Patients.
|
Patient’s Risks
|
Gender (Female)- n (%)
|
Age Under 40, n (%)
|
Mean Age (y)
|
| High risk patients in the placebo group (SD) (n = 60) |
44 (73.3) |
22 (36.67) |
51.91 (± 18.301) |
| High risk patients in the drug group (SD) (n = 74) |
55 (74.32) |
19 (25.68) |
52.834 (± 17.822) |
|
P value |
0.896 |
0.171 |
0.3 |
| 95% CI |
0.827–1.241 |
0.42–1.167 |
51.91 ± 3.288 |
52.834 ± 3.029 |
| RR |
1.013 |
0.7 |
— |
Table 3.
Indications of ERCP in Each Group of Patients
|
Indications
|
Placebo Group
(n=135)
|
Magnesium Group
(n=135)
|
95% CI
|
RR
|
P
Value
|
Total, n (%)
|
| CBD stone with or without cholangitis, n (%) |
82 (60.7) |
83 (61.5) |
0.836–1.224 |
1.012 |
0.9 |
165 (61.1) |
| Periampullary tumor, n (%) |
28 (20.7) |
17 (12.6) |
0.349–1.055 |
0.607 |
0.077 |
45 (16.7) |
| Cholangiocarcinoma, n (%) |
6 (4.4) |
10 (7.4) |
0.623–4.456 |
1.666 |
0.308 |
16 (5.9) |
| CBD stricture (trauma, surgery, bile leak, billoma, undetermined), n (%) |
9 (6.7) |
11 (8.1) |
0.523–2.854 |
1.222 |
0.642 |
20 (7.4) |
| Parasites, n (%) |
4 (3.0) |
2 (1.5) |
0.093–2.684 |
0.5 |
0.418 |
6 (2.2) |
| Metastasis, n (%) |
3 (2.2) |
4 (3.0) |
0.304–5.844 |
1.333 |
0.702 |
7 (2.6) |
| Carolli disease, n (%) |
1 (0.7) |
2 (1.5) |
0.183–21.796 |
2 |
0.569 |
3 (1.1) |
| PSC, n (%) |
2 (1.5) |
4 (3.0) |
0.372–10.737 |
2 |
0.418 |
6 (2.2) |
| Pancreatic duct stone- n (%) |
0 (0) |
2 (1.5) |
0.242–103.186 |
5 |
0.297 |
2 (0.7) |
RR, Relative risk; CBD, Common bile duct; PSC, Primary sclerosing cholangitis.
Table 4.
Pancreatitis Incidence and Severity and their Relation with Risk Assessment of PEP in Each Group of Patients.
|
PEP Characteristics
|
Placebo Group
(n=135)
|
Magnesium Group
(n=135)
|
95% CI
|
OR
|
Total
(n=270)
|
P
Value
|
|
PEP Incidence
|
| Post-ERCP-Pancreatitis n (%) |
17 (12.6) |
12 (8.9) |
0.31–1.48 |
0.68 |
29 (10.7) |
0.33 |
|
Median of hospitalization days
|
|
|
3 |
2 |
(25%percentile-75%percentile) Placebo
2–3 |
(25%percentile-75%percentile) Magnesium
2–2 |
0.04 |
|
PEP severity
|
|
Post-ERCP- Pancreatitis Severity
|
Placebo Group with PEP
(
n
=17)
|
Magnesium Group with PEP
(
n
=12)
|
95% CI
|
OR
|
Total with PEP (n=29)
|
P
Value
|
| Mild, n (%) |
14 (82.4) |
12 (100) |
0.86 |
NaN-infinity |
26 (89.6) |
0.19 |
| Moderate, n (%) |
3 (17.6) |
0 (0) |
0 |
0-NaN |
3 (10.3) |
| Severe, n (%) |
0 (0) |
0 (0) |
NaN |
NaN-NaN |
0 (0) |
|
PEP Incidence in High Risk Patients
|
|
Post-ERCP-Pancreatitis, n (%)
|
Placebo group
(No. of High Risks=60)
(No. of Low Risks=75)
|
Magnesium group
(No. of High Risks=74)
(No. of Low Risks=61)
|
95% CI
|
OR
|
Total
(No. of High Risks=134)
(No. of Low Risks=136)
|
P
Value
|
| Incidence of PEP in high risks, n (%) |
16 (26.67) |
8 (10.81) |
1.18–7.61 |
3 |
24 (17.9) |
0.017 |
| Incidence of PEP in low risks, n (%) |
1 (1.3) |
4 (6.56) |
0.02–1.77 |
0.019 |
5 (3.6) |
0.26 |
PEP, Post-ERCP-Pancreatitis; ERCP, Endoscopic retrograde cholangiopancreatography.
Table 5.
Logistic Regression of Risk Factors
|
Variables
|
Compared Characteristic
|
SE
|
Odds Ratio
|
95% CI
|
P
Value
|
| Trial |
Drug vs placebo |
0.4124 |
0.56 |
0.2494-1.2556 |
0.16 |
| Risk |
High risk vs low risk |
0.5127 |
6.13 |
2.2468-16.7625 |
0.0004 |
| Trial × risk |
Drug & high risk |
0.4747 |
0.33 |
0.1315-0.8452 |
0.02 |
SE, standard error; NaN, Not a Number (Null data).
Discussion
We can elaborately see in Table 4 that 12.6% of patients in the placebo group and 8.9% of patients in the MS group had PEP which revealed no statistically significant difference. MS was not able to prevent PEP in all patients enrolled in this study, but it could significantly reduce the incidence of PEP in high risk patients. In addition, the severity of PEP was not found to be decreased in high risk patients of the MS group in contrast to placebo. There was not a significant difference in terms of age and gender between the two groups of patients. The aggregate percentage of PEP in this study was 10.7%. The total percentage of pancreatitis is a little higher than that reported by other studies which is about 3–10%.1-3 This difference is partially explainable by the number of procedures which were done by fellows of an educational center. Comparable to our study, there are some studies demonstrating a range of 1.6–15% for PEP.4,23 As an instance, a study by Fujita et al on the efficacy of intravenous Flurbiprofen in reduction of PEP reported a total percentage of 11% for PEP.24 Different percentages in several studies would be explicable by various characteristics of patients, risk factors and also the endoscopists’ experience. The severity of PEP in the MS group was mild in 100% of patients; also, the severity of PEP in the placebo group was mild in 14 patients out of 17 patients with PEP (82.4%) and moderate in 3 patients out of 17 patients with PEP (17.6%). The severity of PEP was not statistically different between patients receiving placebo or MS. No severe case of PEP was reported in either group. Although this study did not result in a significant decrease in the number and severity of PEP for patients receiving the drug in comparison with those receiving placebo, it showed an effective reduction in the rate of PEP in high risk patients. In comparison with conclusions drawn from other studies that show the incidence of PEP in high risk patients at approximately 17–40%,22 our study demonstrates similar results for patients in the placebo group but much fewer PEPs in the MS group. Overlooking the interaction effect of being high risk for PEP on the efficacy of MS would result in a big bias. If we had not considered the effect of high risk factors on the performance of MS, no statistically significant difference would have been detected between using the drug or placebo in terms of PEP frequency. When the effect of another variable, i.e. being high risk for PEP is taken into account, the difference becomes significant. However, there is still a question if the lack of efficacy of MS for all patients and low risk patients in this study might be attributed to the low dose of MS and the small sample size in this study or not. Another study is conducted in Germany and Hungary on 502 patients. The dose of MS is 4930 mg in that study. Additionally, the length of hospital stay was found to be lower in PEP patients of the MS group in contrast to the placebo group and must be taken into account although the comparison between 2 days and 3 days may not be logical. The rationale for this result is the number of patients with high risk characteristics for PEP in the MS group which had a significantly lower incidence of PEP and consequently, shorter hospital stay. Despite the possibility of performing ERCPs as an outpatient procedure in private centers, public and educational hospitals are encouraged to hospitalize patients for at least 24 hours and reevaluate patients afterwards for post-ERCP complications. So, the length of hospital stay may be meaningless for centers with an outpatient ERCP protocol but valuable for centers that have a hospitalization protocol after ERCP. There is a lack of publications on the efficacy of MS in reduction of PEP. So, making a comparison with results from other studies is not precisely possible. More studies in different patient groups, with different dosages of this drug and with variable schedules for administration are required to extrapolate these results to others. Additionally, a study design with stratification of serum level of magnesium ions before and after drug injection is recommended to precisely detect if serum magnesium level would affect the reduction of the severity and incidence of PEP. It seems that more studies with larger sample size are required to better approximate the real population of patients needing ERCP. To extrapolate these results to all patients requiring ERCP, it is suggested to consider more long-term studies in different centers with different levels of expertise as well as various ethnic groups of patients.
In conclusion,prescribing 2 g of intravenous MS 1 hour prior to and 6 hours after ERCP can reduce the incidence of PEP in high risk patients but not its severity. More studies about MS with larger sample sizes and different protocols of prescription (higher doses and different schedules of administration) are recommended to make a definite conclusion.
Acknowledgements
This research has been designed and managed under supervision of medical faculty of Tehran University and all expenses were supposed to be provided by research institute budget of TUMS. We show our sincere appreciation to colleagues of the gastroenterology ward of Imam Khomeini hospital who provided us with their insight and expertise in ERCP which were significantly helpful. We thank the nurses and other staff of the endoscopic and gastroenterology ward of Imam Khomeini hospital for assistance with this project.
Authors’ Contribution
NA: Coordinator of the study and first author. HH: Corresponding author and fellowship of ERCP. AA: Statistical analysis. PKM: Data gathering, edition and analysis.
Conflict of Interest Disclosures
All authors declare that they have no conflict of interest.
Ethical Statement
To be in line with moral principles, this study was designed and performed under supervision of Iranian Registry of Clinical Trials [IRCTID: IRCT20180310039017N1 (https://www.irct.ir/trial/30177)] and was reviewed based on 1964 Helsinki Declaration. All the procedures and equipment in this study were supported by the budget of gastroenterology and hepatology research center of Tehran University of medical sciences. All participants in the study were demanded to read and sign a written informed consent if they are consciously agreed upon participation in the study.
References
- Tumi A, Magadmi M, Elfageih S, Rajab AF, Azzabi M, Elzouki AN. ERCP in a cohort of 759 cases: a 6-year experience of a single tertiary centre in Libya. Arab J Gastroenterol 2015; 16(1):25-8. doi: 10.1016/j.ajg.2015.02.004 [Crossref] [ Google Scholar]
- Talukdar R. Complications of ERCP. Best Pract Res Clin Gastroenterol 2016; 30(5):793-805. doi: 10.1016/j.bpg.2016.10.007 [Crossref] [ Google Scholar]
- Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH. Adverse events associated with ERCP. Gastrointest Endosc 2017; 85(1):32-47. doi: 10.1016/j.gie.2016.06.051 [Crossref] [ Google Scholar]
- Shah T, Zfass A, Schubert ML. Chemoprevention of post-ERCP pancreatitis with rectal NSAIDs: does poking both ends justify the means?. Dig Dis Sci 2015; 60(10):2863-4. doi: 10.1007/s10620-015-3746-1 [Crossref] [ Google Scholar]
- Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007; 102(8):1781-8. doi: 10.1111/j.1572-0241.2007.01279.x [Crossref] [ Google Scholar]
- El Nakeeb A, El Hanafy E, Salah T, Atef E, Hamed H, Sultan AM. Post-endoscopic retrograde cholangiopancreatography pancreatitis: risk factors and predictors of severity. World J Gastrointest Endosc 2016; 8(19):709-15. doi: 10.4253/wjge.v8.i19.709 [Crossref] [ Google Scholar]
- Pezzilli R, Romboli E, Campana D, Corinaldesi R. Mechanisms involved in the onset of post-ERCP pancreatitis. JOP 2002; 3(6):162-8. [ Google Scholar]
- Akashi R, Kiyozumi T, Tanaka T, Sakurai K, Oda Y, Sagara K. Mechanism of pancreatitis caused by ERCP. Gastrointest Endosc 2002; 55(1):50-4. doi: 10.1067/mge.2002.118964 [Crossref] [ Google Scholar]
- Akshintala VS, Hutfless SM, Colantuoni E, Kim KJ, Khashab MA, Li T. Systematic review with network meta-analysis: pharmacological prophylaxis against post-ERCP pancreatitis. Aliment Pharmacol Ther 2013; 38(11-12):1325-37. doi: 10.1111/apt.12534 [Crossref] [ Google Scholar]
- Ding J, Jin X, Pan Y, Liu S, Li Y. Glyceryl trinitrate for prevention of post-ERCP pancreatitis and improve the rate of cannulation: a meta-analysis of prospective, randomized, controlled trials. PLoS One 2013; 8(10):e75645. doi: 10.1371/journal.pone.0075645 [Crossref] [ Google Scholar]
- Schick V, Scheiber JA, Mooren FC, Turi S, Ceyhan GO, Schnekenburger J. Effect of magnesium supplementation and depletion on the onset and course of acute experimental pancreatitis. Gut 2014; 63(9):1469-80. doi: 10.1136/gutjnl-2012-304274 [Crossref] [ Google Scholar]
- Park SH, Kim EJ, Chung IK, Kim HS, Lee MH, Kim SJ. Effect of intraduodenal spray of magnesium sulfate MgSO4) on the manometry of sphincter of Oddi. Gastrointest Endosc 2000; 51(4 Pt 2):AB240. doi: 10.1016/s0016-5107(00)14670-x [Crossref] [ Google Scholar]
- Saunders JH, Thjodleifsson B, Wormsley KG. Effect of intraduodenal magnesium sulphate on pancreas and gallbladder of man. Gut 1976; 17(6):435-8. doi: 10.1136/gut.17.6.435 [Crossref] [ Google Scholar]
- Tammaro S, Caruso R, Pallone F, Monteleone G. Post-endoscopic retrograde cholangio-pancreatography pancreatitis: is time for a new preventive approach?. World J Gastroenterol 2012; 18(34):4635-8. doi: 10.3748/wjg.v18.i34.4635 [Crossref] [ Google Scholar]
- Zhang ZF, Duan ZJ, Wang LX, Zhao G, Deng WG. Aggressive hydration with lactated ringer solution in prevention of postendoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis of randomized controlled trials. J Clin Gastroenterol 2017; 51(3):e17-e26. doi: 10.1097/mcg.0000000000000781 [Crossref] [ Google Scholar]
- Cheon YK. Can postendoscopic retrograde cholangiopancreatography pancreatitis be prevented by a pharmacological approach?. Korean J Intern Med 2013; 28(2):141-8. doi: 10.3904/kjim.2013.28.2.141 [Crossref] [ Google Scholar]
- Li X, Tao LP, Wang CH. Effectiveness of nonsteroidal anti-inflammatory drugs in prevention of post-ERCP pancreatitis: a meta-analysis. World J Gastroenterol 2014; 20(34):12322-9. doi: 10.3748/wjg.v20.i34.12322 [Crossref] [ Google Scholar]
- Patil S, Pandey V, Pandav N, Ingle M, Phadke A, Sawant P. Role of rectal diclofenac suppository for prevention and its impact on severity of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Gastroenterology Res 2016; 9(2-3):47-52. doi: 10.14740/gr672w [Crossref] [ Google Scholar]
- Rainio M, Lindström O, Udd M, Louhimo J, Kylänpää L. Diclofenac does not reduce the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis in low-risk units. J Gastrointest Surg 2017; 21(8):1270-7. doi: 10.1007/s11605-017-3412-3 [Crossref] [ Google Scholar]
- Manes G, Ardizzone S, Lombardi G, Uomo G, Pieramico O, Porro GB. Efficacy of postprocedure administration of gabexate mesylate in the prevention of post-ERCP pancreatitis: a randomized, controlled, multicenter study. Gastrointest Endosc 2007; 65(7):982-7. doi: 10.1016/j.gie.2007.02.055 [Crossref] [ Google Scholar]
- Uchino R, Isayama H, Tsujino T, Sasahira N, Ito Y, Matsubara S. Results of the Tokyo trial of prevention of post-ERCP pancreatitis with risperidone-2: a multicenter, randomized, placebo-controlled, double-blind clinical trial. Gastrointest Endosc 2013; 78(6):842-50. doi: 10.1016/j.gie.2013.06.028 [Crossref] [ Google Scholar]
- Anikhindi SA, Kumar A, Singla V, Sharma P, Bansal N, Verma N. The revised Atlanta classification is the strongest predictor of mortality in patients with acute pancreatitis: a study on 358 Patients. Trop Gastroenterol 2020; 40(4):137-50. doi: 10.7869/tg.555 [Crossref] [ Google Scholar]
- Fluhr G, Mayerle J, Weber E, Aghdassi A, Simon P, Gress T. Pre-study protocol MagPEP: a multicentre randomized controlled trial of magnesium sulphate in the prevention of post-ERCP pancreatitis. BMC Gastroenterol 2013; 13:11. doi: 10.1186/1471-230x-13-11 [Crossref] [ Google Scholar]
- Fujita Y, Hasegawa S, Kato Y, Ishii K, Iwasaki A, Sato T. Intravenous injection of low-dose flurbiprofen axetil for preventing post-ERCP pancreatitis in high-risk patients: an interim analysis of the trial. Endosc Int Open 2016; 4(10):E1078-E82. doi: 10.1055/s-0042-115172 [Crossref] [ Google Scholar]