Arch Iran Med. 24(9):689-695.
doi: 10.34172/aim.2021.99
Original Article
Serum Expression of IL-33 and ST2 in Patients with Psoriasis Vulgaris
Yonghua Dong 1  , Hua Hu 1, Dandan Fu 1, Shuting Zheng 1, Qingqing Wang 1, Keshav K C 1, Xiangfeng Song 2, Zhongwei Tian 1, *
, Hua Hu 1, Dandan Fu 1, Shuting Zheng 1, Qingqing Wang 1, Keshav K C 1, Xiangfeng Song 2, Zhongwei Tian 1, * 
Author information:
1Department of Dermatology, The First Affiliated Hospital of Xinxiang Medical University, Henan, Xinxiang 453000, China
2Department of Immunology, Xinxiang Medical University, Henan, Xinxiang 453000, China
*Corresponding Author: Zhongwei Tian, MD; Department of Dermatology, The First Affiliated Hospital of Xinxiang Medical University, Henan, Xinxiang 453000, China. Tel: +86-0373-4402634; Email:
zhonwt@xxmu.edu.cn
Abstract
Background:
Psoriasis vulgaris (PsV) is an immune-mediated skin disease of unknown mechanism. Interleukin 33 (IL-33) is a member of IL-1 cytokine family and suppression of tumorigenicity 2 (ST2) is the specific ligand of IL-33. It has been found that IL-33 and ST2 are increased in psoriatic lesions, but the expression levels in serum and their relationship to clinical features are still unclear. The aim of this study is to assess IL-33, ST2, IL-17 and IL-5 serum levels as well as serum concentration of blood glucose and blood lipids in PsV patients and their relationship with clinical characteristics.
Methods:
Sixty-eight PsV samples and 60 healthy individuals were recruited. Serum levels of IL-33, ST2, IL-17 and IL-5 were measured by enzyme-linked immunosorbent assay and blood glucose and blood lipid were assayed by automatic biochemical analyzer.
Results:
Serum levels of IL-33, ST2, IL-17 and IL-5 were increased significantly in PsV patients compared with controls (P < 0.01). Cytokines were overexpressed in PsV patients during active stages compared with controls (P < 0.05). Expression levels of IL-33, ST2 and IL-17 confirmed a significance in different severity groups of PsV patients (P < 0.05). Serum concentration of triglyceride (TG) was also increased compared with controls (P = 0.024). IL-33 levels were positively correlated with total cholesterol (TC) levels (r=0.319, P = 0.008).
Conclusion:
IL-33/ST2 could generally reflect the activity and disease severity in PsV patients, which indicates that the IL-33/ST2 signaling pathway plays an important role in the pathogenesis of PsV.
Keywords: Interleukin-33, Psoriasis Vulgaris, Severity, Stages, Tumor Suppressor
Copyright and License Information
© 2021 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article as: Dong Y, Hu H, Fu D, Zheng S, Wang Q, KC K, et al. Serum expression of il-33 and st2 in patients with psoriasis vulgaris. Arch Iran Med. 2021;24(9):689-695. doi: 10.34172/aim.2021.99
Introduction
Psoriasis Vulgaris (PsV) is a kind of chronic inflammatory and immune-mediated skin disease which causes great psychological pressure and economic burden for the patients.1 Its prevalence is about 2%-3% worldwide.2 PsV is not only a simple skin disease, but compared with healthy people, the patients are also more likely to develop other systemic disease conditions like psoriatic arthritis, metabolic syndrome, non-alcoholic fatty liver disease, Crohn’s disease, cardiovascular disease, anxiety and depression.3 The typical clinical manifestations of PsV are erythema, scaling and infiltration on the skin. Although the exact pathological mechanism of psoriasis is still unclear, it is considered to be related to autoimmune, hereditary, infectious and environmental factors.4 A study including 78 626 women demonstrated that overweight and adiposity are also dangerous factors for the course of psoriasis.5 The main pathological mechanism of psoriasis is the overproliferation and dysfunctional differentiation of keratinocytes and acanthosis. There is a large number of inflammatory cells in psoriasis lesions, including T cells, neutrophils, dendritic cells and macrophages.4 Some evidence suggests that many cytokines and chemokines are involved in the pathomechanism of psoriasis, such as interleukin-17 (IL-17), IL-5, interferon alpha (IFN-α), tumor necrosis factor alpha (TNF-α), IL-23, IL-12, CXCL9 and CXCL11. Among them, IL-17, IL-23 and TNF-α are considered to be the key cytokines in the process of occurrence and development of psoriasis.6
IL-33, a new member of the IL-1 cytokine family, can be expressed in a variety of organs and cells in the human body, such as the skin, epithelial cells, endothelial cells, fibroblasts, macrophages, dendritic cells, smooth muscle cells, stomach, lung, central nervous system and lymph nodes.7 As an “alarm”, IL-33 can be released from injured and stressed cells to send out warning of cellular necrosis or tissue damage to the innate and adaptive immune systems.8 Suppression of tumorigenicity 2 (ST2) is the receptor of IL-33, which is encoded by the IL1RL1 gene. ST2 is identified in 3 splicing variants in human: a transmembrane receptor (ST2L), a released soluble form (sST2) and a variant of ST2 (ST2V). The sST2 can act as a decoy receptor and has anti-inflammatory properties.9 The combination of IL-33 and ST2 can promote the release of many cytokines and chemokines so as to participate in the pathological process of various diseases including psoriasis, atopic dermatitis, vitiligo and lung diseases.10
Previously, we showed that IL-33/ST2 were increased in the skin of psoriasis-like mouse models, which aggravated disease severity in mice.11 There is less data on the clinical correlation with serum IL-33/ST2 levels in PsV patients to date; therefore, our study aimed to analyze serum IL-33, ST2, IL-17 and IL-5 levels as well as serum concentrations of blood glucose (Glu), blood lipids like total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) in PsV patients and the relationship with clinical characteristics in order to provide a new target for treatment of psoriasis.
Materials and Methods
Patients and Controls
Sixty-eight PsV patients [39 (57.4%) men and 29 (42.7%) woman, age 29.34 ± 11.20 years, range 18-62] were selected from the Department of Dermatology and Venereal diseases in the First Affiliated Hospital of Xinxiang Medical University from September 2018 to April 2019. The diagnosis and stages of PsV were mainly based on the Clinical Dermatology by Bian Zhao [Active stage, 43 (63.2%) cases; Stationary stage, 11 (16.2%) cases; Retrograde, 14 (20.6%) cases]. The psoriasis area and severity index (PASI) score was calculated according to different location of lesions (including head, upper limb, trunk and lower limb) and extent of erythema, scaling and infiltration on the skin. The PsV group was divided into three subgroups according to the PASI score: mild [PASI score < 3, 27 (39.7%) cases], moderate (PASI score 3-10, 33 (48.5%) cases) and severe (PASI score ≥ 10, 8 (11.8%) cases). In addition, family history and recent infection history were also recorded (Table 1). Another 60 healthy people [37 (61.7%) men and 23 (38.3%) woman, age 31.82 ± 12.63 years, range 18-62] without a family history of psoriasis and autoimmune diseases were recruited as normal controls. No statistically significance was observed for age (t = 1.47, P = 0.228) or gender distribution (c2 = 0.246, P = 0.620) between the psoriasis and control groups (Table 1).
Table 1.
Baseline Demographics, Clinical Characteristics and Laboratory Data of Study Groups.
|
Variables
|
PsV (n = 68)
|
Controls (n = 60)
|
Total Number (n = 128)
|
c2 & U/T Value
|
P
value
|
| Gender (n [%]) |
|
|
| Male |
39 [57.4%] |
37 [61.7%] |
76 [59.4%] |
0.246 |
0.620 |
| Female |
29 [42.7%] |
23 [38.3%] |
52 [40.6%] |
| Age (y) (Mean ± SD) |
29.34 ± 11.20 |
31.82±12.63 |
30.50 ± 11.91 |
1.470 |
0.228 |
| Family history (n [%]) |
|
|
|
|
|
| Yes |
17 [25.0%] |
0 [0%] |
17 [13.3%] |
17.297 |
< 0.001 |
| No |
51 [75.0%] |
60 [100%] |
111 [86.7%] |
| Infection history (n [%]) |
|
|
|
|
|
| Yes |
28 [41.2%] |
0 [0%] |
28 [21.9%] |
31.624 |
< 0.001 |
| No |
40 [58.8%] |
60 [100%] |
100 [78.1%] |
| Stages (n [%]) |
|
|
|
|
|
| Active |
43 [63.2%] |
0 [0%] |
— |
— |
— |
| Stationary |
11 [16.2%] |
0 [0%] |
— |
| Retrograde |
14 [20.6%] |
0 [0%] |
— |
| PASI score (n [%]) |
|
|
|
|
|
| Gently (0–3) |
27 [39.7%] |
0 [0%] |
— |
— |
— |
| Middle (3–10) |
33 [48.5%] |
0 [0%] |
— |
| Severe (≥10) |
8 [11.8%] |
0 [0%] |
— |
| Biochemical indicators (Mean ± SD) |
|
|
|
|
|
| Glu (mmol/L) |
5.62 ± 1.50 |
5.35 ± 0.61 |
5.49 ± 1.17 |
0.616 |
0.538 |
| TC (mmol/L) |
4.06 ± 0.76 |
3.91 ± 0.70 |
3.99 ± 0.74 |
0.838 |
0.402 |
| TG (mmol/L) |
1.31 ± 0.49 |
1.18 ± 0.65 |
1.25 ± 0.57 |
2.257 |
0.034 |
| HDL (mmol/L) |
1.33 ± 0.24 |
1.37 ± 0.27 |
1.35 ± 0.25 |
0.418 |
0.608 |
| LDL (mmol/L) |
2.17 ± 0.57 |
2.05 ± 0.56 |
2.11 ± 0.56 |
1.032 |
0.302 |
PsV, psoriasis vulgaris; PASI, psoriasis area and severity index; Glu, glucose; TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; SD, standard deviation.
Sample and Measures
The sample size of psoriasis patients was calculated by parameters including typeⅠerror at 5%, power of test at 90% and d = 5.21, minimal clinically relevant difference and variance at 3.97 according to the results of a study in China.12 In addition, assuming 10% attrition, the sample size was determined to be 68 psoriasis patients and 60 healthy individuals in the study after strict inclusion and exclusion criteria.
Samples of peripheral venous blood from PsV patients and control group were collected in the morning and then incubated for 15-30 minutes at room temperature. Blood serum was isolated by centrifugation for 5 minutes at 4000r/min, and frozen at -80℃ until further analysis. Serum levels of IL-33, ST2, IL-17 and IL-5 were measured by enzyme-linked immunosorbent assay (ELISA) (Shanghai Enzyme-linked Biotechnology Co, Ltd, Shanghai, China) according to manufacturer’s direction. The blood glucose and blood lipids were detected by Beckman automatic biochemical analyzer in the First Affiliated Hospital of Xinxiang Medical University.
Statistical Analysis
Statistical analysis of the data was performed using the Statistical Package for Social Sciences (SPSS) software, version 22.0. Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were by described through frequency and percentage. Classified variables were analyzed by chi-square test, and the large expected cell counts of chi-square test were also checked. Normality was evaluated using skewness, kurtosis, and normal Q-Q plots and Kolmogorov-Smirnov test with a P value < 0.05 indicating a non-normally distributed population. If the data satisfied normal distribution and homogeneity of variance, Student’s t test and ANOVA were used to analyze different groups; otherwise, Mann-Whitney U test and Kruskal-Wallis H test were used. Spearman correlation test was used to analyze the relationship between the cytokine levels and blood glucose and blood lipids. A value of P ≤ 0.05 was considered statistically significant.
Results
Serum IL-33, ST2 IL-17 and IL5 Levels in PsV Patients and Controls
The serum levels of IL-33 (31.72 ± 28.36 versus 19.99 ± 10.56 pg/mL, P < 0.001), ST2 (174.03 ± 107.82 versus 121.24 ± 48.57 pg/mL, P = 0.024), IL-17 (72.40 ± 48.53 versus 48.84 ± 21.60 pg/mL, P = 0.001), and IL-5 (19.94 ± 8.26 versus 15.26 ± 5.81 pg/mL, P < 0.001) were increased in PsV patients compared with controls (Figure 1).
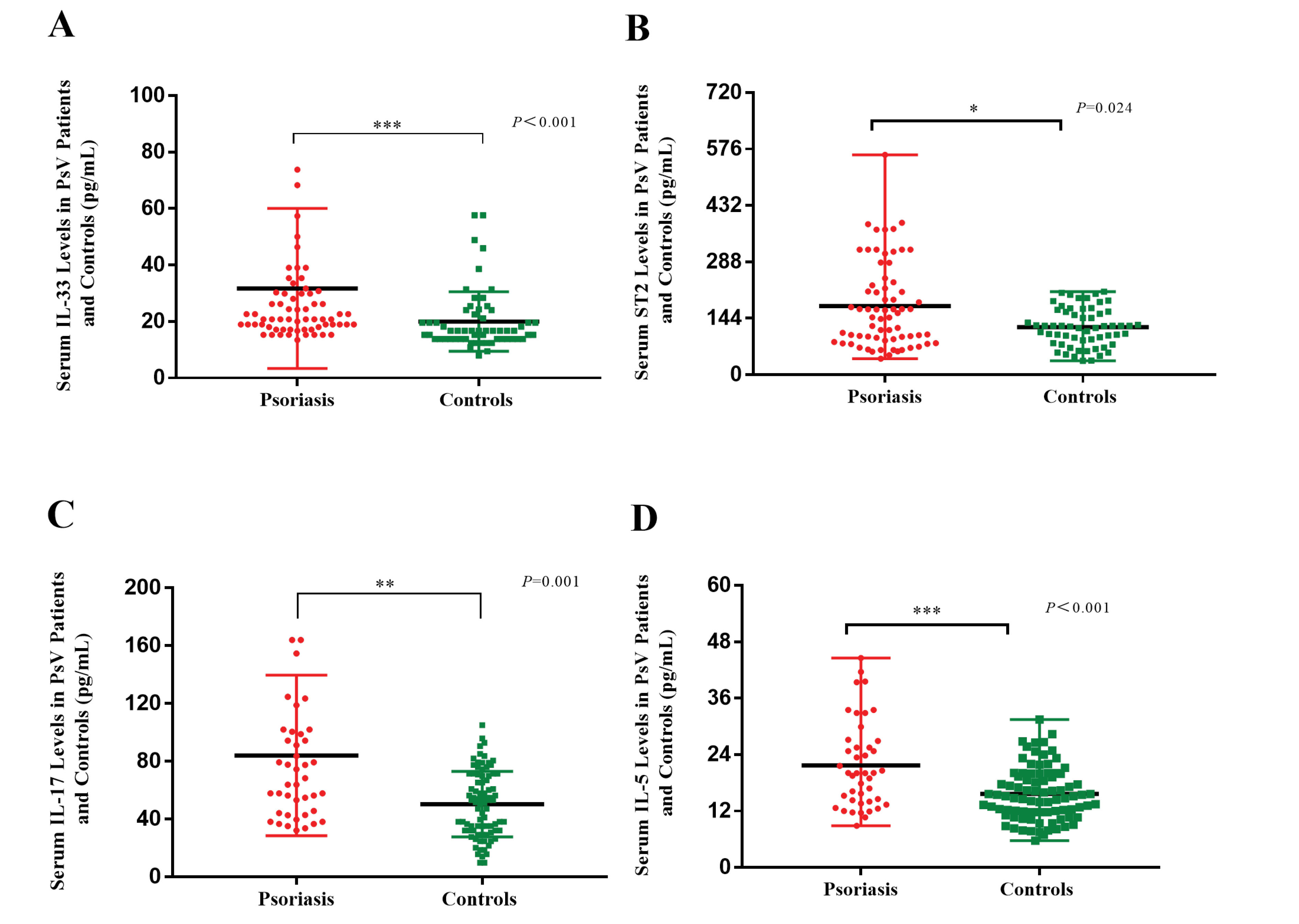
Figure 1.
Serum IL-33, ST2, IL-17 and IL-5 Levels in PsV Patients and Controls. The Mann-Whitney test was used in the comparisons. *P < 0.05; **P < 0.01; ***P < 0.001.
.
Serum IL-33, ST2, IL-17 and IL-5 Levels in PsV Patients and Controls. The Mann-Whitney test was used in the comparisons. *P < 0.05; **P < 0.01; ***P < 0.001.
Serum IL-33, ST2, IL-17 and IL-5 Levels in Different Severities of PsV Patients
Levels of IL-33 (57.68 ± 52.17 versus 22.39 ± 7.54 pg/mL, P = 0.011) and IL-17 (130.27 ± 97.83 versus 55.55 ± 27.22 pg/mL, P = 0.033) were increased significantly in the severe group compared with those in the mild group; ST2 level (194.82 ± 106.88 versus 130.78 ± 75.48 pg/mL, P = 0.032) in the moderate group was higher differently than the mild group. There was no significant difference between other groups (Figure 2).
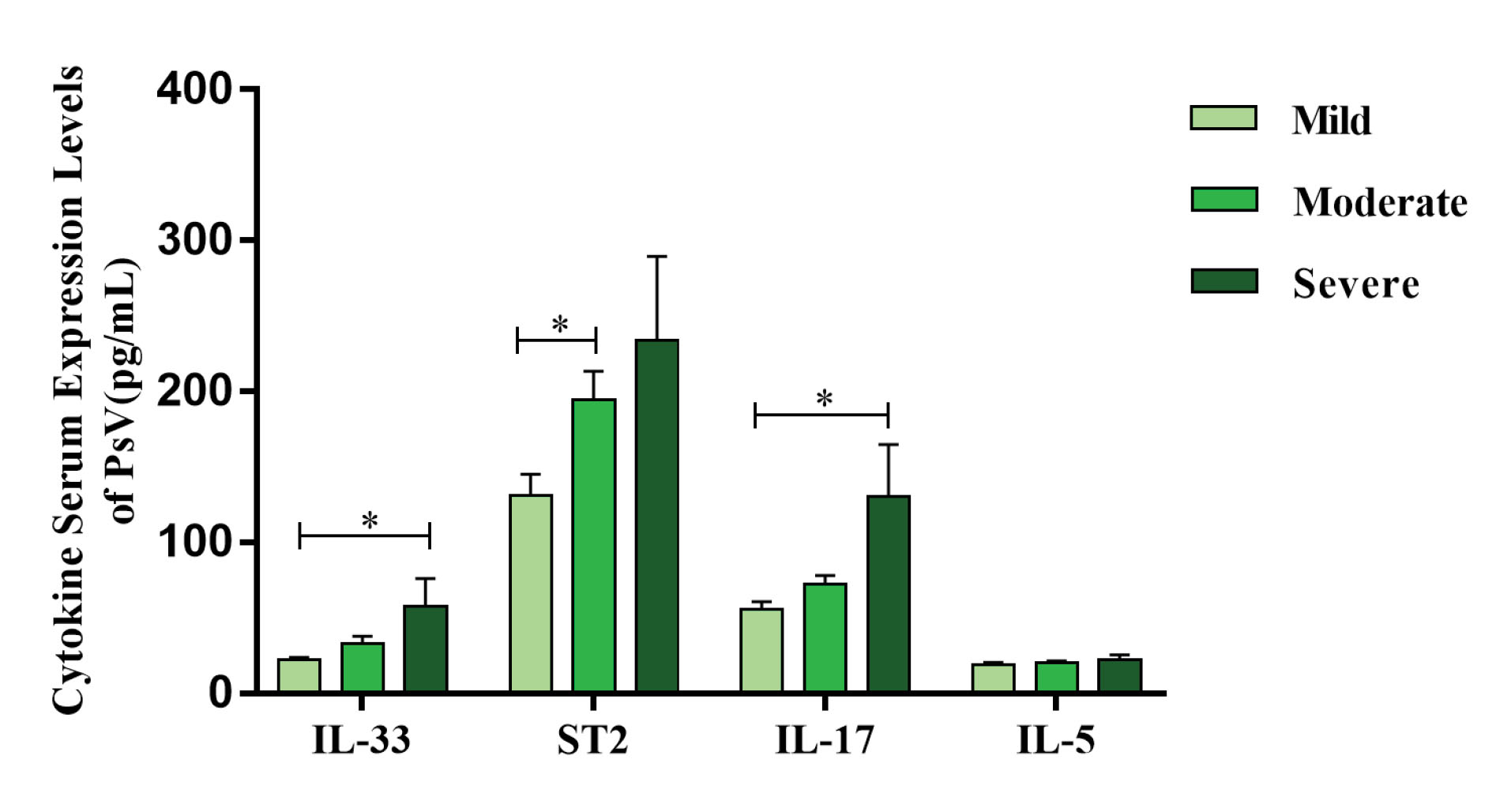
Figure 2.
Serum IL-33, ST2, IL-17 and IL-5 Levels in Different Severities of PsV Patients. The Kruskal-Wallis H test was used in comparisons. *P < 0.05.
.
Serum IL-33, ST2, IL-17 and IL-5 Levels in Different Severities of PsV Patients. The Kruskal-Wallis H test was used in comparisons. *P < 0.05.
Serum IL-33, ST2, IL-17 and IL-5 Levels in Different Stages of PsV Patients
Serum levels of IL-33 (36.99 ± 34.07 versus 19.99 ± 10.56 pg/mL, P < 0.001), ST2 (193.01 ± 111.28 versus 121.24 ± 48.57 pg/mL, P = 0.009), IL-17 (83.98 ± 55.54 versus 48.84 ± 21.60 pg/mL, P < 0.001) and IL-5 (21.85 ± 9.23 versus 15.26 ± 5.81 pg/mL, P < 0.001) in active PsV patients were significantly higher than controls. However, only IL-17 (83.98 ± 55.54 versus 47.22 ± 21.59 pg/mL, P = 0.022) and ST2 (193.01 ± 111.28 versus 113.95 ± 81.96 pg/mL, P = 0.009) serum levels in the active stage were significantly increased compared with those in the retrograde stage in PsV patients. No significant difference was observed between other groups (Figure 3).
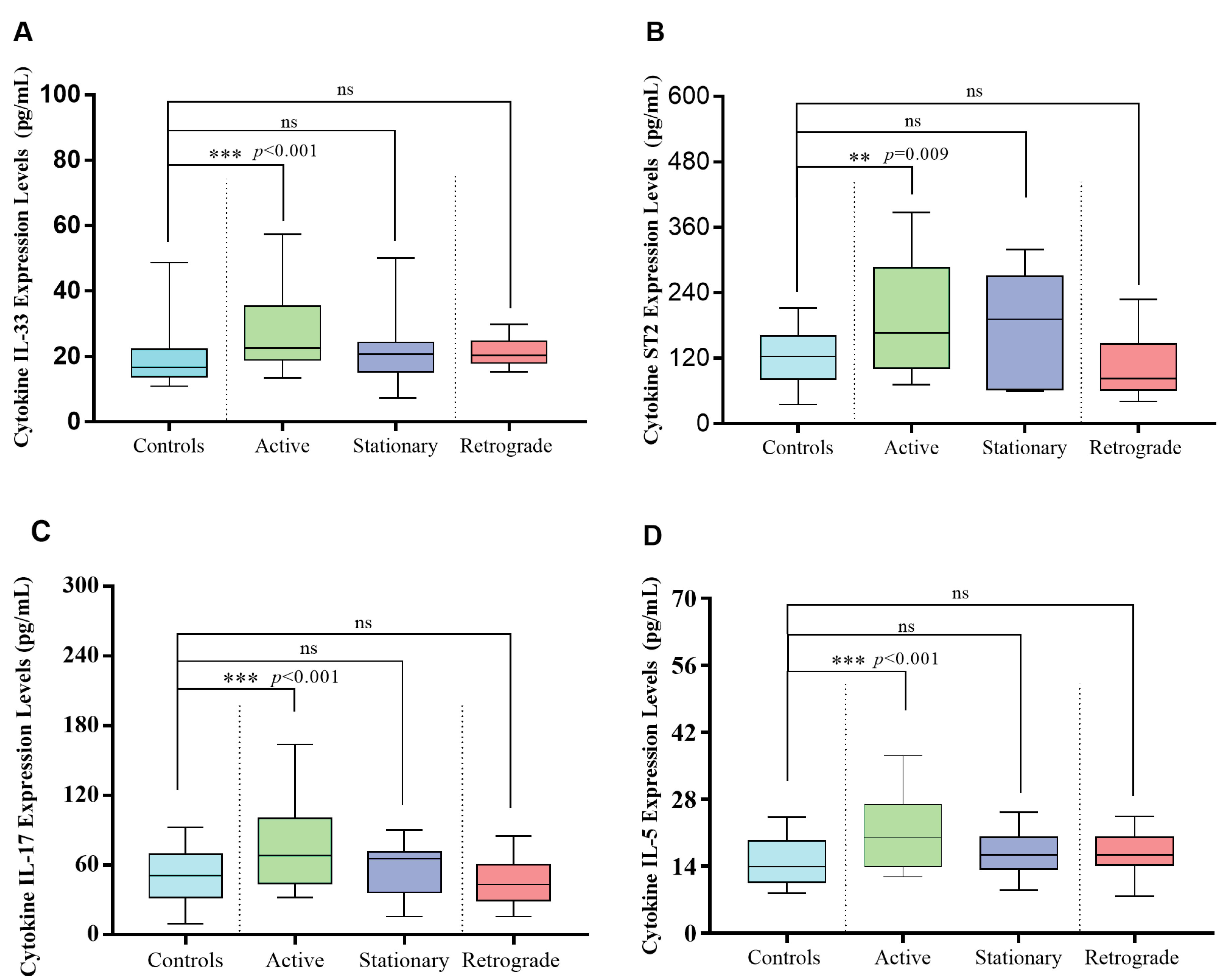
Figure 3.
Serum IL-33, ST2, IL-17 and IL-5 Levels in PsV Patients for Different Stages and Controls. The Kruskal-Wallis H test was used in comparisons.*P < 0.05; **P < 0.01;***P < 0.001; ns means no significant difference.
.
Serum IL-33, ST2, IL-17 and IL-5 Levels in PsV Patients for Different Stages and Controls. The Kruskal-Wallis H test was used in comparisons.*P < 0.05; **P < 0.01;***P < 0.001; ns means no significant difference.
Serum Glu, TC, TG, HDL and LDL Concentrations in PsV Patients
Serum concentration of TG was increased significantly in PsV patients (P = 0.024). No significant difference was observed for Glu, TC, HDL and LDL between PsV patients and controls (Table 1).
Correlations of IL-33, ST2, IL-17 and IL-5 Serum Levels with Glu, TC, TG, HDL and LDL in PsV Patients
Serum levels of IL-33 showed a significant correlation with serum TC concentrations (r = 0.319, P = 0.008). However, there was no significant correlation in other groups (Figure 4).
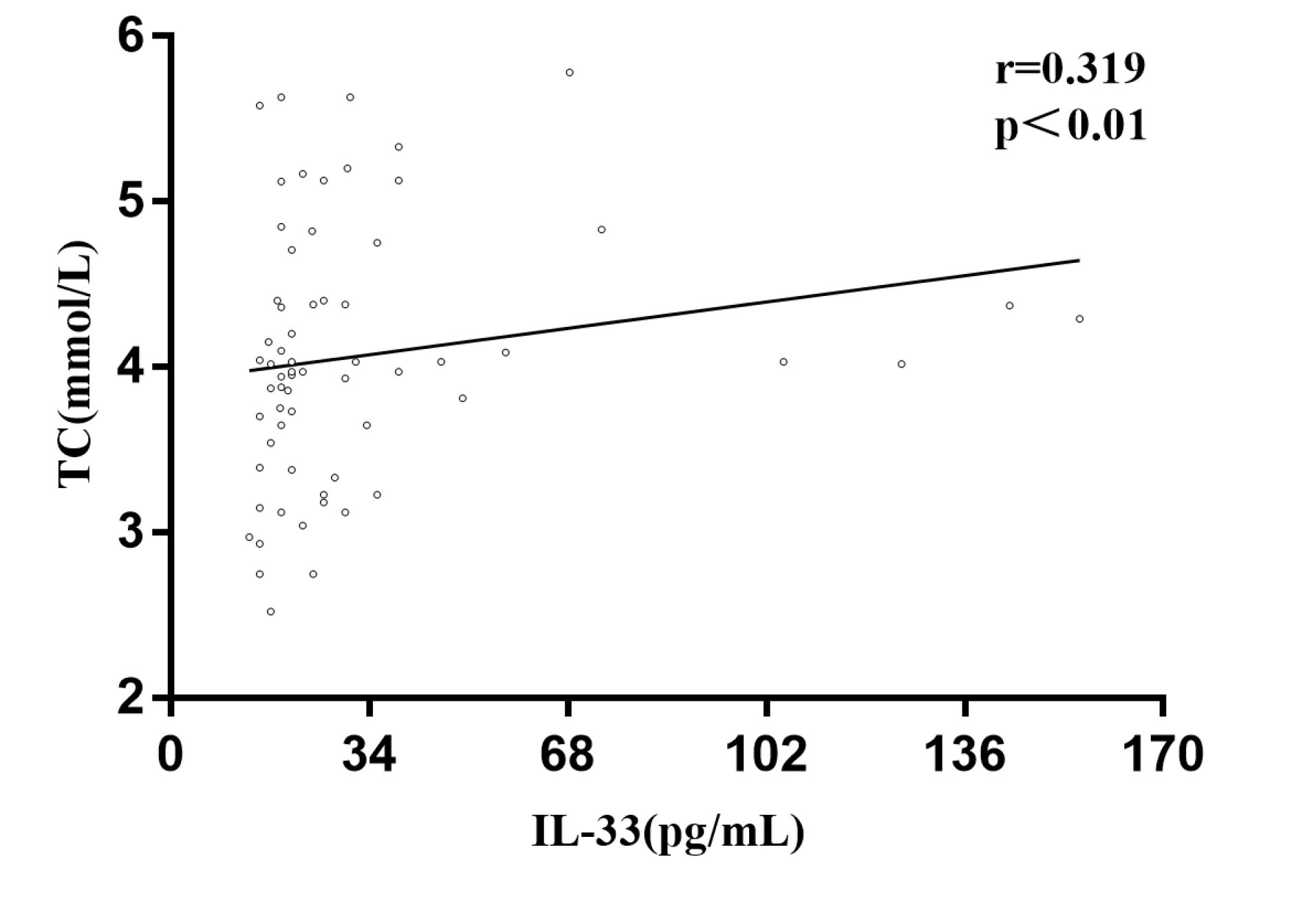
Figure 4.
Correlation between Serum Levels of IL-33 and TC in PsV Patient.
.
Correlation between Serum Levels of IL-33 and TC in PsV Patient.
Discussion
Psoriasis vulgaris is considered to be a mixed Th17/Th1 disease with strong IFN-γ and IL-17 signatures. IL-17 induces the proliferation of keratinocytes. It can also promote the production of CXCL1, CXCL2, CXCL8 and IL-36 in keratinocytes. These factors stimulate neutrophil migration to the epidermis, thus playing a crucial role in the development of psoriasis.13 Th17 and Th1 cells are also present in the skin tissue affected by psoriasis. Kagam et al used single-color flow analysis and found that circulating IL-17 was increased in psoriasis patients.14 In our study, serum levels of IL-17 were significantly higher in PsV patients than controls, which is also in agreement with the results of Caproniet al.15 IL-17 inhibitors are used to treat psoriasis and improve the condition of PsV patients, and their tolerability, safety and efficacy have been shown for the treatment of psoriasis.16
IL-5 is produced mainly by CD4+ Th2 cells, eosinophils, mast cells and basophils. Recombinant human IL-5 has the ability to activate eosinophils induced reactive oxygen species and kill antibody-coated target cells.17,18 Solberg et al analyzed the changes in serum IL-5 levels after 16 weeks of treatment with biological agents and found that IL-5 expressions were increased at follow-up but only a few patients contributed to this increase. At the same time, there was a moderate correlation between IL-5 and the percentage change of PASI, which indicated that IL-5 contributed to the pathological mechanism of psoriasis.6 Our data suggested that IL-5 serum levels increased in the PsV patients, and its concentration in the active stage was higher compared to controls and different PsV stages. However, we did not find different expressions of serum IL-5 in mild, moderate and severe groups of PsV patients, which indicates that there is no association between IL-5 and the severity of psoriasis. This is contrary to the study by Solberget al, which may be due to the small sample size in the severe group.
IL-33 was firstly identified in 2005 by Jochen Schimitz.19 IL-33 can enhance the effect of peptide substance P on release of vascular endothelial growth factor from mast cells, and increase vascular permeability and promote inflammatory response. The interaction between IL-33 and ST2 may play an important role in inflammatory diseases with excessive vascular hyperplasia, such as psoriasis.20 Balato et al found that IL-33 was significantly increased in psoriasis lesions and non-skin lesions. Immunogold staining showed that IL-33 was expressed in the nucleus and cytoplasm of keratinocytes, as well as at the junction of keratinocytes, indicating that keratinocytes can secrete IL-33 in psoriasis. However, the circulating levels of IL-33 was not detected in psoriasis.21 A previous study has shown that IL-33 increased in the serum of patients with psoriasis, especially in psoriatic arthritis, but no correlation was observed between serum IL-33 levels and PASI.22 Subsequently, although Sehat et al found a significant correlation between serum levels of IL-33 and disease severity, there was no significant difference in IL-33 levels between patients with psoriasis and control group.23 Our data showed that IL-33 levels were significantly higher in PsV patients than controls and related to the activity and severity of PsV, which confirms that IL-33 contributes to the pathological mechanisms of PsV during the active stage. A previous study showed that IL-33 can inhibit the autophagy-associated proteins by promoting the phosphorylation of STAT3 in psoriasis-like mouse models, so IL-33 may combine with ST2 to promote the production of inflammatory cells by activating the STAT3 in keratinocytes, thus affecting the severity of the disease during the active stage of psoriasis.11 Meanwhile, our results showed a positive correlation between serum levels of IL-33 and TC levels. Zeyda et al found that severe obesity was associated with elevated IL-33 and its receptor ST2 expression in endothelial cells of the adipose tissue in both humans and mice.24 Weight reduction can improve pre-existing psoriasis or psoriasis arthritis and prevent the onset of psoriasis in obese individuals.25 Our results suggested that IL-33 may increase the severity of psoriasis by promoting obesity, but the exact and complex interaction mechanism between IL-33, obesity and psoriasis is still unclear, and needs further study.
To our knowledge, ST2, the receptor of IL-33, can be expressed by some immune cells involved in type 2 immune response, such as two groups of innate lymphoid cells, Th2 cells, mast cells, basophils eosinophils and dendritic cells.26 Hueber et al used phorbol ester to induce a mouse model and found that ST2 -/- mice had a smaller epidermis than WT mice. ST2 -/- mice also exhibited weak skin inflammatory response compared to WT mice.27 We confirmed that ST2 serum levels were increased in PsV patients compared with controls and higher in the active stage than controls and the retrograde group. The result may demonstrate that ST2 is associated with activity of PsV and may be involved in the pathogenesis of psoriasis. Our study also showed that ST2 was expressed differently in mild, moderate and severe groups, with the highest content in the severe group, suggesting that ST2 may contribute to the severity of PsV. A previous study has reported that ST2 levels was elevated in humans with obesity, and there was a positive association between ST2 levels and different risk factors for developing diabetes after adjusting for age and sex, indicating that increased ST2 levels can promote diabetes.24,28 However, we did not find an association between ST2 expression and blood glucose and lipid, which may probably reflect the complex role of immune and metabolic factors in the pathogenesis of psoriasis where many mechanisms still need to be clarified.
The relationship between dyslipidemia and PsV is also unclear. Previous studies have shown that serum TG concentrations may be normal or increased whereas TC levels may be increased, normal or decreased in patients with psoriasis. The expression levels of HDL and LDL were also different. Panget al found that serum levels of TC, LDL, HDL were higher in controls than those with psoriasis, but there was no significant difference in TG between the two groups.29 However, no significant difference was observed for Glu, TC, HDL and LDL levels between PsV patients and controls in our study. We found a significant difference between PsV and controls in terms of serum TG concentration. Metabolic syndrome includes hyperglycemia, obesity, elevated blood pressure and dyslipidemia, which are closely related to psoriasis. As the number of metabolic syndrome components increased, the risk of psoriasis was also increased.30 Increased serum TG concentration is more likely to be involved in the risk of PsV compared with Glu, TC and LDL.
In conclusion, the strength of our study is the relationship between serum IL-33/ST2 levels in PsV and clinical features, demonstrating that IL-33/ST2 contribute to the pathological mechanism of psoriasis, and have a correlation with activity and severity of PsV. How IL-33/ST2 affects the occurrence and development of PsV and whether we can improve the condition of PsV by controlling serum IL-33, ST2 and TG levels require further study. In this study, we did not adjust the confounding factors and this may have affected the results, which is a limitation of the study. In addition, we collected samples and information of PsV patients. We did not collect enough samples of patients with erythrodermic psoriasis, arthropathic psoriasis and pustular psoriasis, which may be another shortcoming of this study.
Acknowledgements
This work was supported by the Technology Innovation Talents Project of Henan Health and Family Planning Commission (grant number 201632), the Combined Construction Project of Health Commission of Henan Province (grant number LHGJ20190469), The Graduate Student Innovation Support Plan of Xinxiang Medical University (grant number YJSCX201919Z).
Authors’ Contribution
YD: Data collection, statistical analysis, manuscript drafting. HH, DF, SZ, QW and KK: Data collection. XS and ZT: Design and critical revision.
Conflict of Interest Disclosures
The authors have no conflicts of interest.
Ethical Statement
The study was approved by the Ethics Committee of the First Affiliated Hospital of Xinxiang Medical University (No:2019298). Everyone All participants in this experiment were well informed and consent was taken.
References
- Lebwohl M. Psoriasis. Ann Intern Med 2018; 168(7):ITC49-ITC64. doi: 10.7326/aitc201804030 [Crossref] [ Google Scholar]
- Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol 2001; 26(4):314-20. doi: 10.1046/j.1365-2230.2001.00832.x [Crossref] [ Google Scholar]
- Boehncke WH, Schön MP. Psoriasis. Lancet 2015; 386(9997):983-94. doi: 10.1016/s0140-6736(14)61909-7 [Crossref] [ Google Scholar]
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci 2019; 20(6):1475. doi: 10.3390/ijms20061475 [Crossref] [ Google Scholar]
- Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Intern Med 2007; 167(15):1670-5. doi: 10.1001/archinte.167.15.1670 [Crossref] [ Google Scholar]
- Solberg SM, Sandvik LF, Eidsheim M, Jonsson R, Bryceson YT, Appel S. Serum cytokine measurements and biological therapy of psoriasis - prospects for personalized treatment?. Scand J Immunol 2018; 88(6):e12725. doi: 10.1111/sji.12725 [Crossref] [ Google Scholar]
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 2010; 10(2):103-10. doi: 10.1038/nri2692 [Crossref] [ Google Scholar]
- Liu X, Xiao Y, Pan Y, Li H, Zheng SG, Su W. The role of the IL-33/ST2 axis in autoimmune disorders: friend or foe?. Cytokine Growth Factor Rev 2019; 50:60-74. doi: 10.1016/j.cytogfr.2019.04.004 [Crossref] [ Google Scholar]
- De la Fuente M, MacDonald TT, Hermoso MA. The IL-33/ST2 axis: role in health and disease. Cytokine Growth Factor Rev 2015; 26(6):615-23. doi: 10.1016/j.cytogfr.2015.07.017 [Crossref] [ Google Scholar]
- Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol 2017; 8:475. doi: 10.3389/fimmu.2017.00475 [Crossref] [ Google Scholar]
- Duan Y, Dong Y, Hu H, Wang Q, Guo S, Fu D. IL-33 contributes to disease severity in psoriasis-like models of mouse. Cytokine 2019; 119:159-67. doi: 10.1016/j.cyto.2019.02.019 [Crossref] [ Google Scholar]
- Dong Z. Expression and Significance of IL-31, IL-33 and CCL27 in The Serum of Patients with Psoriasis Vulgaris. Zhengzhou: Zhengzhou University; 2018.
- Furue M, Kadono T. The contribution of IL-17 to the development of autoimmunity in psoriasis. Innate Immun 2019; 25(6):337-43. doi: 10.1177/1753425919852156 [Crossref] [ Google Scholar]
- Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 2010; 130(5):1373-83. doi: 10.1038/jid.2009.399 [Crossref] [ Google Scholar]
- Caproni M, Antiga E, Melani L, Volpi W, Del Bianco E, Fabbri P. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trial. J Clin Immunol 2009; 29(2):210-4. doi: 10.1007/s10875-008-9233-0 [Crossref] [ Google Scholar]
- Ly K, Smith MP, Thibodeaux Q, Reddy V, Liao W, Bhutani T. Anti IL-17 in psoriasis. Expert Rev Clin Immunol 2019; 15(11):1185-94. doi: 10.1080/1744666x.2020.1679625 [Crossref] [ Google Scholar]
- Menzella F, Lusuardi M, Galeone C, Taddei S, Zucchi L. Profile of anti-IL-5 mAb mepolizumab in the treatment of severe refractory asthma and hypereosinophilic diseases. J Asthma Allergy 2015; 8:105-14. doi: 10.2147/jaa.s40244 [Crossref] [ Google Scholar]
- Dougan M, Dranoff G, Dougan SK. GM-CSF, IL-3, and IL-5 family of cytokines: regulators of inflammation. Immunity 2019; 50(4):796-811. doi: 10.1016/j.immuni.2019.03.022 [Crossref] [ Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005; 23(5):479-90. doi: 10.1016/j.immuni.2005.09.015 [Crossref] [ Google Scholar]
- Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A 2010; 107(9):4448-53. doi: 10.1073/pnas.1000803107 [Crossref] [ Google Scholar]
- Balato A, Lembo S, Mattii M, Schiattarella M, Marino R, De Paulis A. IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp Dermatol 2012; 21(11):892-4. doi: 10.1111/exd.12027 [Crossref] [ Google Scholar]
- Mitsui A, Tada Y, Takahashi T, Shibata S, Kamata M, Miyagaki T. Serum IL-33 levels are increased in patients with psoriasis. Clin Exp Dermatol 2016; 41(2):183-9. doi: 10.1111/ced.12670 [Crossref] [ Google Scholar]
- Sehat M, Talaei R, Dadgostar E, Nikoueinejad H, Akbari H. Evaluating serum levels of IL-33, IL-36, IL-37 and gene expression of IL-37 in patients with psoriasis vulgaris. Iran J Allergy Asthma Immunol 2018; 17(2):179-87. [ Google Scholar]
- Zeyda M, Wernly B, Demyanets S, Kaun C, Hämmerle M, Hantusch B. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int J Obes (Lond) 2013; 37(5):658-65. doi: 10.1038/ijo.2012.118 [Crossref] [ Google Scholar]
- Mahil SK, McSweeney SM, Kloczko E, McGowan B, Barker JN, Smith CH. Does weight loss reduce the severity and incidence of psoriasis or psoriatic arthritis? a critically appraised topic. Br J Dermatol 2019; 181(5):946-53. doi: 10.1111/bjd.17741 [Crossref] [ Google Scholar]
- Lu J, Kang J, Zhang C, Zhang X. The role of IL-33/ST2L signals in the immune cells. Immunol Lett 2015; 164(1):11-7. doi: 10.1016/j.imlet.2015.01.008 [Crossref] [ Google Scholar]
- Hueber AJ, Alves-Filho JC, Asquith DL, Michels C, Millar NL, Reilly JH. IL-33 induces skin inflammation with mast cell and neutrophil activation. Eur J Immunol 2011; 41(8):2229-37. doi: 10.1002/eji.201041360 [Crossref] [ Google Scholar]
- Lin YH, Zhang RC, Hou LB, Wang KJ, Ye ZN, Huang T. Distribution and clinical association of plasma soluble ST2 during the development of type 2 diabetes. Diabetes Res Clin Pract 2016; 118:140-5. doi: 10.1016/j.diabres.2016.06.006 [Crossref] [ Google Scholar]
- Pang X, Lin K, Liu W, Zhang P, Zhu S. Characterization of the abnormal lipid profile in Chinese patients with psoriasis. Int J Clin Exp Pathol 2015; 8(11):15280-4. [ Google Scholar]
- Kim HN, Han K, Park YG, Lee JH. Metabolic syndrome is associated with an increased risk of psoriasis: a nationwide population-based study. Metabolism 2019; 99:19-24. doi: 10.1016/j.metabol.2019.07.001 [Crossref] [ Google Scholar]